DNA Melting: Simulating DNA Melting - Basics
Introduction
Chance favors the prepared mind.
—Louis Pasteur (abridged)
This tutorial will show you how to simulate the DNA melting experiment with Matlab. The DNA melting apparatus you will build produces two output voltages related to the sample temperature and fluorescence intensity. During an experimental run, these two voltages will be recorded periodically. At the end of this tutorial, you will be able to produce mock datasets that are similar to what you will record in the lab. You can use simulated datasets to help develop and debug your data analysis scripts.
It is essential to have working data analysis scripts before you begin running experiments in the lab.
The procedure for creating the simulation is:
- Write a function to compute the equilibrium dsDNA concentration vs. temperature
- Model sample temperature versus time during an experimental run
- Compute the RTD voltage from temperature vs. time data
- Compute relative fluorescence intensity vs. time data from temperature vs. time data
Run Matlab and follow along as you read. Code from this page can be copied and pasted into the Matlab command window.
If you prefer to work in Python, please see this page.
Before you begin
- Review the DNA Melting Thermodynamics Notes.
- If you need a review of Matlab fundamentals, see Matlab Fundamentals tutorial.
Matlab skills
- Array arithmetic (element-by-element) operators (+, -, .*, ./)
- Functions declared in an m-file
- Conditionals
- Functions that take a variable number of arguments
- Numerical differentiation
- Plotting data
Modeling dsDNA equilibrium fraction
We derived an expression for dsDNA fraction at a given temperature in class:
- $ K_{eq} = e^\left [\frac{\Delta S^{\circ}}{R} - \frac{\Delta H^{\circ}}{R T} \right ] $
- $ f = \frac{1 + C_T K_{eq} - \sqrt{1 + 2 C_T K_{eq}}}{C_T K_{eq}} $
where $ C_T $ is the total concentration of DNA strands.
Since this is a complicated equation that we will evaluate many times, it makes sense to write a Matlab function.
dsDNA fraction function
The fraction of dsDNA is a function of 4 variables: ΔS°, ΔH°, temperature, and concentration. The DnaFraction function below also takes an optional fifth argument for the gas constant to enable using different unit systems. Using the element-by-element divide operator in the body of the function makes it possible for DnaFraction to accept either a single temperature argument or a vector of temperature values. (As a general rule, use the ./ operator instead of / unless you intend to do a matrix divide.)
The Matlab function nargin returns the number of arguments that the function was called with. Many Matlab programs use this function to handle variable numbers of arguments. If nargin returns 4 or less, R is set to the default value of 1.987. Otherwise, the value of R is set to the value supplied by the caller.
Matlab notes: Matlab functions must be saved in the current directory or another directory in the Matlab path. The name of the file must be the function name appended with ".m". Function help should be placed in comments at the top of the file. Type help DnaFraction to see how this works.
% Returns the fraction of dsDNA in a solution containing equal concentrations
% of two complementary DNA oligos as a functino of total DNA concentration,
% temperature, entropy change, and enthalpy change.
% Gas constant is an optional parameter.
%
% USAGE: f = DnaFraction(Ct, T, DeltaS, DeltaH, <GasConstant>)
% RETURN VALUE: fraction of dsDNA
%
% Default units are molar, Kelvin, cal/mole, and cal/(mole-Kelvin). For
% other unit systems, supply the appropriate value of R in the optional
% fifth parameter. The default value is 1.987 cal/(degree K * mole).
function f = DnaFraction(Ct, T, DeltaS, DeltaH, GasConstant)
% Gas constant
if(nargin < 5) % determine if caller supplied R value or not
R = 1.987; % set to default: cal/(degree K * mole)
else
R = GasConstant; % otherwise use caller's value
end
% Compute Ct * Keq
CtKeq = Ct * exp(DeltaS / R - DeltaH ./ (R * T));
%now compute f
f = (1 + CtKeq - sqrt(1 + 2 * CtKeq)) ./ CtKeq;
% Written 4/9/2008 by SCW
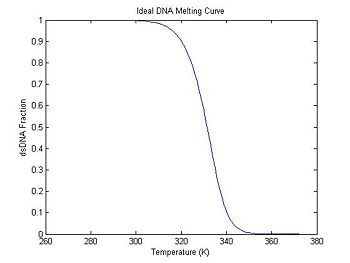
Plot an ideal melting curve
Does the DnaFraction function work as expected? A good way to test the function is to plot a melting curve generated by DnaFraction.
Matlab notes: Over the course of an interactive Matlab session, you may run several simulations with different parameters. Using a structure to keep the results of each simulation together is a good way to prevent the workspace from becoming disorganized. The struct function creates an empty structure. In the code below, a structure called ideal is created to hold the ideal melting curve data. (Since variables are dynamically allocated and typed in Matlab, the struct command is optional; however, explicitly creating the structure can make your code easier to read and better performing.)
Since DnaFraction accepts a vector temperature argument, an entire melting curve can be computed with a single call.
close all;
clc;
ideal = struct(); % create an empty structure to hold the results
ideal.temperature = (0:99) + 273.15; % temperature axis in Kelvin: 100 points(0-99C)
ideal.deltaS = -184; % cal/mol
ideal.deltaH = -71E3; % cal/(mol K)
ideal.DnaConcentration = 30E-6; % M, ssDNA concentration (each strand)
% call DnaFraction function
% arguments: concentration, temperature vector, delta S, delta H
% (optional R argument not supplied)
% DnaFraction uses total DNA concentration = 2 x ssDNA concentration
ideal.dsDnaFraction = DnaFraction(2*ideal.DnaConcentration, ...
ideal.temperature, ideal.deltaS, ideal.deltaH);
figure(1)
plot(ideal.temperature, ideal.dsDnaFraction);
title('Ideal DNA Melting Curve');
xlabel('Temperature (K)');
ylabel('dsDNA Fraction');
Experiment with different values of ΔS° and ΔH° to see how the curve changes.
Several web tools are available to predict the melting temperature as well as ΔS° and ΔH°. See, for example, DINA Melt or Oligocalc. You can use one of these or another tool to estimate values for different DNA sequences. The DNA sequences used in the lab are listed at DNA Melting: DNA Sequences. For DNA Melting Part I, you will be using a 20 base pair sequence ("20bp" and its complement "20bp-comp") at 30μM concentration in a 100mM saline solution. Try plugging in the 20bp sequence (or other sequence listed) into DINA Melt and/or Oligocalc to get estimates of the thermodynamic parameters.
One method of estimating Tm is to find the temperature where the dsDNA fraction equals 1/2. Another method is to differentiate the melting curve. Tm occurs at the peak of the derivative — where the dsDNA fraction is changing the fastest. (Why might you prefer one method over the other when estimating Tm from experimental data?)
Numerical differentiation
The fluorescence function fN is a sampled version of the continuous function f(T) such that fN = f(NΔT), where ΔT is the sampling interval (1° C, in this instance). One method for computing a numerical approximation to df/dT is the finite difference:
- $ {f'}_N = \frac{f_{N+1}-f_{N}}{\Delta T} $.
df/dT is guaranteed to take on the value given by f'N at some point between NΔT and (N+1)ΔT; however, this happy coincidence can occur at any place in the interval. A simple but effective technique is to guess that equality occurs in the middle of the interval. This guess is close enough in most cases.
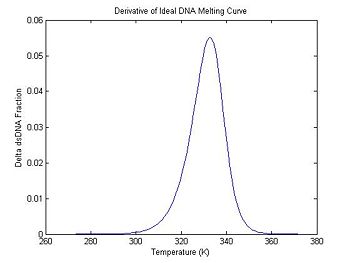
The code below computes the finite difference and stores it in the dDsDnaFraction field of ideal. It also generates an associated temperature vector (ideal.dTemperature) with values centered between the original temperature samples. Note that both vectors are one element shorter than the original data.
ideal.dTemperature = ideal.temperature(1:99) + 0.5; % create new temperature vector
ideal.dDsDnaFraction = diff(ideal.dsDnaFraction); % take adjacent element differences
figure(2)
plot(ideal.dTemperature, -1 * ideal.dDsDnaFraction);
title('Derivative of Ideal DNA Melting Curve');
xlabel('Temperature (K)');
ylabel('Delta dsDNA Fraction');
In the previous section, the plot shows that the dsDNA fraction equals 0.5 at about 332.5 degrees. The peak of the derivative also occurs at the same temperature. (The curve is plotted inverted.)
Set up simulation conditions and data structure
It will be convenient to set up a data structure with the simulation parameters and a time axis.
sim1 = struct(); % create empty struct for sim results sim1.DnaConcentration = 30E-6; % 30 micromolar concentration sim1.deltaS = ideal.deltaS; % cal / (mole-K) sim1.deltaH = ideal.deltaH; % cal / mole sim1.finalTemperature = 293; % approx. room temperature sim1.sampleRate = 1; % 1 Hz sample rate sim1.durationHT = 900; % heating time, s sim1.durationCL = 600; % cooling time, s sim1.timeHT = (0:(1/sim1.sampleRate):sim1.durationHT)'; % heating time vector, s sim1.timeCL = (1:(1/sim1.sampleRate):sim1.durationCL)'; % cooling time vector, s sim1.time = [sim1.timeHT; sim1.timeHT(end)+sim1.timeCL]; % complete time vector, s
Simulate sample temperature during an experimental run
The goal of the simulation is to produce two simulated voltage signals that resemble what you will measure in the lab. Since both of the voltages are functions of temperature, it makes sense to begin by modeling how the sample temperature behaves during an experimental run.
Sample heating and cooling
During each experimental run, the range of temperatures will be provided by forced heating by a TEC, followed by natural cooling of the sample after the heat source is switched off. At the beginning of an experimental run the sample will be at room temperature, then the TEC is switched on and the DNA + fluorescent dye sample is heated to 95°C in about 5 minutes. Upon reaching 95°C, the TEC power is switched off. Then over a period of about 15 minutes, the sample cools to room temperature (less than 30°C). The rate of cooling is proportional to the difference between the sample's temperature and the room temperature, so the sample cools more quickly at the start of the experiment than near the end. (It can be frustrating to wait for the final few degrees.)
Both heating and cooling can be approximated by an exponential function. Heating is modeled with a time constant of about 1 minute, while cooling is modeled with a time constant of about 3 minutes. The equation for temperature (in K) versus time is:
- $ T_{heating}(t) = (T_{max} - T_i) (1 - e ^ {-\frac{t_{HT}}{\tau_{HT}}}) + T_i $
- $ T_{cooling}(t) = (T_{max} - T_f) e ^ {-\frac{t_{CL}}{\tau_{CL}}} + T_f $
where,
- T(t) is the temperature as a function of time,
- Ti is the initial temperature, likely room temperature,
- Tmax is the max temperature reached during the heating phase,
- Tf is the final temperature, likely room temperature,
- τHT and τCL are the time constants for heating (HT) and cooling (CL).
In Matlab:
sim1.heatingConstant = 180; % heating rate constant, 1/s
sim1.coolingConstant = 300; % cooling rate constant, 1/s
sim1.maxTemperature = 95 + 273; % target maximum sample temp, K
sim1.initialTemperature = 20 + 273; % starting room temp, K
sim1.finalTemperature = 20 + 273; % final cool-down temp, K
% use exp function to model exponential heating and cooling
sim1.temperature = ...
[ (sim1.maxTemperature - sim1.initialTemperature) ... % (T_max - T_i) *
.* (1 - exp(-sim1.timeHT ./ sim1.heatingConstant)) ... % (1 + e^(-t/tauHT)) +
+ sim1.initialTemperature; ... % T_i
(sim1.maxTemperature - sim1.finalTemperature) ... % (T_max - T_f) *
.* exp(-sim1.timeCL ./ sim1.coolingConstant) ... % e^(-t/tauCL) +
+ sim1.finalTemperature ]; % T_f
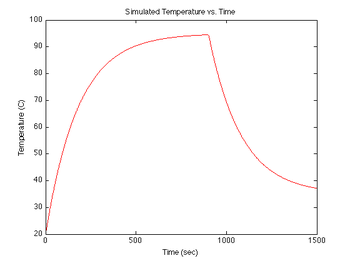
figure(3)
plot(sim1.time, sim1.temperature-273, 'r');
xlabel('Time (sec)');
ylabel('Temperature (C)');
title('Simulated Temperature vs. Time');
If you have any questions about the element-by-element division operator in Matlab (./), or the use of a semicolon for concatenation, see:this page or ask an Instructor or TA.
RTD temperature sensor model
RTD and voltage divider circuit
The resistance of the RTD is given by the equation $ R_{RTD} = R_o*(1 + \alpha \Delta T) $, where "α" is the temperature coefficient of resistance (TCR) for the RTD and $ R_o $ is the resistance at the reference temperature for the RTD. In the experimental apparatus, the RTD is hooked up as one element of a voltage divider with a pull-up resistor, R. The power supply input is 15 volts. Thus, the output voltage is: $ V_{RTD} = 15 * R_{RTD}/(R + R_{RTD}) $. The following code calculates RTD voltage measurements from sim1.temperature and stores the result in sim1.V_RTD, assuming $ R $ = 15kΩ, $ R_o $ = 1000Ω, $ \alpha $ = 3850 ppm/°C, and $ \Delta T $ is the difference, in Kelvin or Celsius, between the RTD reference temperature and the RTD temperature.
sim1.R_RTD_atZeroC = 1000; % Datasheet value of reference RTD resistance, Ω
sim1.RTDalpha = 3850; % Datasheet value of RTD TCR, ppm/C
sim1.R_RTD = sim1.R_RTD_atZeroC ...
* (1 + sim1.RTDalpha*1e-6 * (sim1.temperature-273)); % Compute RTD resistance, Ohms
sim1.R_pullup = 15e3; % Pull-up resistor in voltage divider, Ohms
sim1.V_input = 15; % Input voltage of divider, V
sim1.V_RTD = sim1.V_input * sim1.R_RTD ...
./ (sim1.R_pullup + sim1.R_RTD); % RTD votlage drop, V
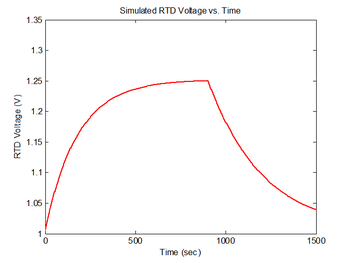
figure(4)
plot(sim1.time, sim1.V_RTD, 'r');
xlabel('Time (sec)');
ylabel('RTD Voltage (V)');
title('Simulated RTD Voltage vs. Time');
Simulate DNA fraction for this temperature profile
Use the DnaFraction function given above to calculate the simulated fluorescence using the combined heating and cooling temperature profile that you just calculated.
sim1.dsDnaFraction = DnaFraction(sim1.DnaConcentration, ...
sim1.temperature, sim1.deltaS, sim1.deltaH);
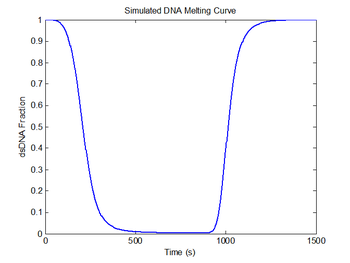
figure(5)
plot(sim1.time, sim1.dsDnaFraction);
title('Simulated DNA Melting Curve');
xlabel('Time (s)');
ylabel('dsDNA Fraction');
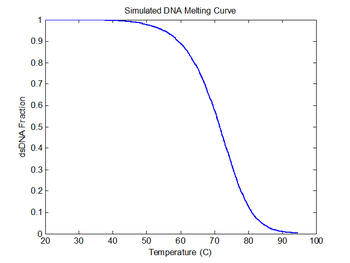
figure(6)
plot(sim1.temperature-273, sim1.dsDnaFraction);
title('Simulated DNA Melting Curve');
xlabel('Temperature (C)');
ylabel('dsDNA Fraction');
| |
For the homework assignment, use either DINA Melt or Oligocalc to determine the ΔS° and ΔH° for a given DNA sequence and use the code above to estimate the melting temperature: |
- Start with one of the DNA sequences listed at DNA Melting: DNA Sequences (one of the 20bp, 30bp, or 40bp sequences and its complement). Use either DINA Melt or Oligocalc to determine the ΔS° and ΔH° of the sequences (at 30μM ssDNA concentration and 100mM salt).
- Plug those thermodynamic parameters into the code listed above and reproduce Figures 4, 5, and 6.
- Estimate the melting temperature Tm as the temperature where the dsDNA fraction equals 1/2 and at the peak of the derivative.
For further work: Add noise to the simulated signals
You will certainly notice noise on the signals you capture in the lab. Noise will be an extremely important (and annoying) factor in this experiment because the melting curves will be differentiated. Differentiation emphasizes noise. In order to ensure that your data analysis effectively handles the noise you are likely to observe, simulated random noise will be added to the signals.
To simulate noise, we can use Matlab's randn function, which generates normally distributed random numbers with unity standard deviation. (The size function is used to tell randn to create a vector of random numbers with the same size (and shape) as V_RTD.)
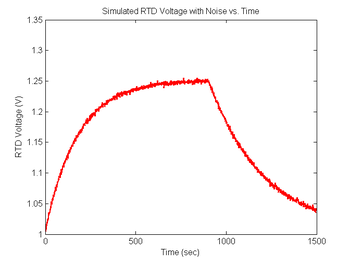
noise = 0.001 * randn(size(sim1.V_RTD)); % Create noise vector, standard deviation=1mV
sim1.V_RTD = sim1.V_RTD + noise; % Add simulated noise to the voltage signal
figure(7)
plot(sim1.time, sim1.V_RTD, 'r');
xlabel('Time (sec)');
ylabel('RTD Voltage (V)');
title('Simulated RTD Voltage with Noise vs. Time');
Now that we have added noise to the RTD voltage signal, the next step is to do the same for the fluorescence signal.
The output range of the transimpedance amplifier depends on many factors, including: dsDNA quantity, illumination intensity, fluorophore efficiency, optical system gain, photodiode efficiency, and electronic amplifier gain. All of the factors except dsDNA concentration essentialy remain constant. Thus, the output of the transimpedance amplifier can be modeled by a single overall system gain factor times the dsDNA fraction. In addition, the amplifier output may be offset by a constant DC value, which is simple to model. (If the output of your apparatus drifts significantly over time, you will need to improve your implementation and methods to minimize this.)
The output range of the transimpedance amplifier over the course of an experimental run can vary depending on several factors, including the amplifier gain, optical system gain, etc... A typical setup might produce values between, say, 0.5 and 7.2 Volts. The simulation code generates two random numbers to set the range and minimum value of the data. Your data analysis script will have to normalize the amplifier output voltage to a fractional value between 0 and 1. (What assumptions will you make about dsDNA concentration to do the normalization?)
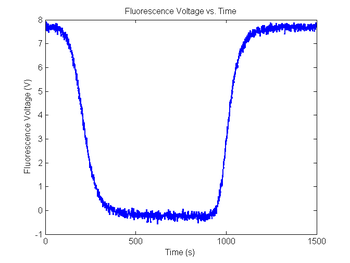
Range = 5 + 4 * rand(); % Range between 5 and 9
Minimum = 2 * rand() - 1; % Minimum between +/- 1
sim1.V_f = Range * sim1.dsDnaFraction + Minimum; % Scale output
noise = 0.01 * randn(size(sim1.V_f)); % Random noise, standard deviation=10mV
sim1.V_f = sim1.V_f + noise; % Add random noise
figure(8)
plot(sim1.time, sim1.V_fNoisy)
title('Fluorescence Voltage vs. Time');
xlabel('Time (s)');
ylabel('Fluorescence Voltage (V)');
Finally, plot the noisy fluorescence data versus the noisy temperature data by converting the noisy RTD voltage to temperature.
sim1.TNoisy = (sim1.R_pullup*sim1.V_RTDNoisy./(sim1.V_input-sim1.V_RTDNoisy) ...
- sim1.R_RTD_atZeroC) / (sim1.RTDalpha*sim1.R_RTD_atZeroC*1e-6) ;
figure(9)
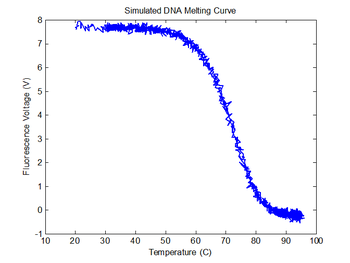
plot(sim1.TNoisy, sim1.V_fNoisy);
title('Simulated DNA Melting Curve');
xlabel('Temperature (C)');
ylabel('Fluorescence Voltage (V)');
You will likely find that you first need to either sort or bin your temperature data so that the fluorescence vs temperature function can be differentiated.