DNA Melting Part 2: Lock-in Amplifier and Temperature Control
Overview of Part 2
In part 1 you constructed the basic components of a precision instrument, learned the basics of its operation, and discovered its limits, of which there are many. Chief among these limits was the heating rate. This limitation showed itself primarily through an inability to deal with an extreme temperature lag between the sample temperature and the measured heating block temperature. Another troublesome limitation was exposed by your instruments' inability to reject various noise or general disturbance sources, such as ambient light in the laboratory, slow variations of that light, and even electrical coupling effects from the passage of another student near your instrument.
In part 2 of the DNA Melting Lab you will improve your instrument by implementing a number of hardware and software changes that will allow you to overcome many of the limitations that you had encountered.
Hardware changes
These are changes that you must make to your instrument. Further details are provided below.
- Review and possibly adjust your optics based on results in Part 1
- Add a heater/cooler board to your heating system.
- Revise your LED driver circuit.
- Revise your high gain, dual-stage amplifier, with filters of your choice.
Software changes
These are changes that have mostly been made for you. Details are included below.
- Implemented a temperature controller in the Labview VI
- Implemented a lock-in amplifier in the Labview VI
- Download a new VI from the DNA Melting folder on the course share
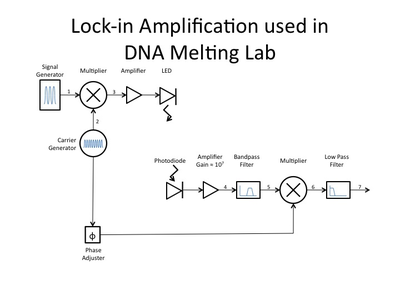
Why use a lock-in amplifier?
Recall from lecture that we will implement the lock-in amplifier to make precision measurements in a noisy environment. The general lock-in amplifier technique can be used to recover a signal from a noisy environment as long as the frequency of that signal is known. We will use this technique to induce a signal of interest at a particular frequency chosen to lie in a low-noise area of the laboratory background noise spectrum. In that we way we might be able to dial in a desired signal to noise ratio, as long as we consider the effects of operating at that frequency on all of the components of our instrument and our specimens.
Opportunities for further development
For those so-inclined, there is much fertile ground for further improvement of both the instrument and our understanding of the physical processes involved. We welcome your interest in pursuing such improvements. Some possibilities are listed here.
- There is another method for controlling the heating profile of your DNA sample. It so happens that the heater/cooler board has been designed as a "shield" for an Arduino controller board. An initial version of software for that purpose has been tested and debugged. One next step that could be to integrate the Arduino controller into the over-all Labview Lock-in VI. See us if you are interested.
- You will fit a multi-parameter model to your data. One primary weakness in our implementation of such a model at this time involves the convective heat loss term in the transfer function of the heating lag model between the block and the sample. We have some ideas to address this. If you do also, or would like to hear more, please see us.
- Also, to do a DNA melting curve properly, one must heat (or cool) the sample extremely slowly. If we tried this with our current approach the sample would be far too bleached by the end. One method to address this would be to revise the Labview VI so that the LED turns only briefly, and only after temperatures have stabilized to eliminate the lab between block and sample. If you are interested in tackling this, please see one of us.
- Next, we do not believe that we have a fully-developed bleaching correction for this experiment. You may agree after trying your hand at this correction. If you would like to investigate further, please see one of us.
- Any and all other ideas for improvement will also be given fair review!
Update your electrical system
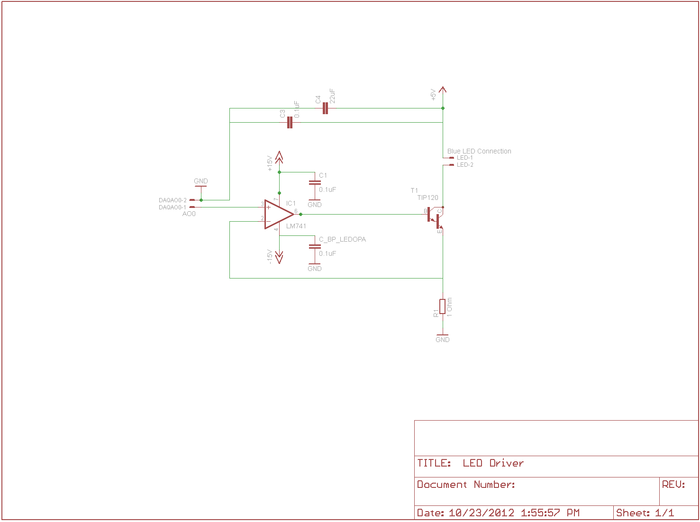
LED driver
The LED used in this lab can consume 1.5 Amps of current when driven to its full range, but this is more than 10x the light you will need. In part 1 of the lab you had used roughly 90 mA, and for part 2 you will likely use an even lower current, nevertheless, the analog voltage output sources in the DAQ systems are only capable of driving very small currents[1][2]. And even if there were enough current available, the steepness of the diode's I-V curve would result in large swings in current for small changes in supply voltage. LED radiant flux — your excitation light — is proportional to current, so we will implement a controlled current source and direct it through the LED to provide a more stable sample excitation.
To implement the current source to drive your LED, you will be using a Darlington Transistor [3]. This is a special device in which one transistor drives another to provide higher input-output current gain. At LED current levels around 100 mA the TIP120 will be providing a current gain of about 500[4]. A transistor [5] is a three terminal device in which the voltage or current at one terminal controls the voltage across, or current through, the other two terminals. In our case, the TIP120 acts like one BJT consisting of a Base, Emitter, and Collector, and should be wired into the circuit as shown in the schematic below. We will use the base contact of our TIP120 to accept a small amount of current from the LM741 op-amp[6], which will cause the TIP120 to conduct a much larger current between it's collector and emitter, in turn allowing nearly the same level of current to flow through the LED.
As described in lecture, the op-amp will be used to linearize the behavior of the TIP120 and the LED. Here the LED and transistor are inside a feedback loop extending from the output of the op-amp, through the base-emitter conduction path in the TIP120, through the LED, and back from the top of the 1 Ohm resistor, whose voltage drop is thus fed-back to the negative input node of the op-amp, completing the loop. Recall that this is simply a buffer implementation, with gain of 1. Thus the feedback voltage is directly proportional to the LED current. Notice that in this way the current through the LED is exactly equal to the voltage applied to the op-amp positive input by the DAQ. (Remember the golden rules.) Therefore we are directly controlling the current through the LED, and likewise the excitation light delivered to our dsDNA specimen.
Note: if the LED is too bright then the sample will become photobleached very quickly. In the Labview VI for Part 2, you are able to set the voltage supplied to the op-amp.
Note also that the voltage to the LED still comes from the fixed 5V supply on your lab station's INSTEK GPS-3303 power supply, while the op-amps still operate on +/– VCC (+/-15V in your setup).
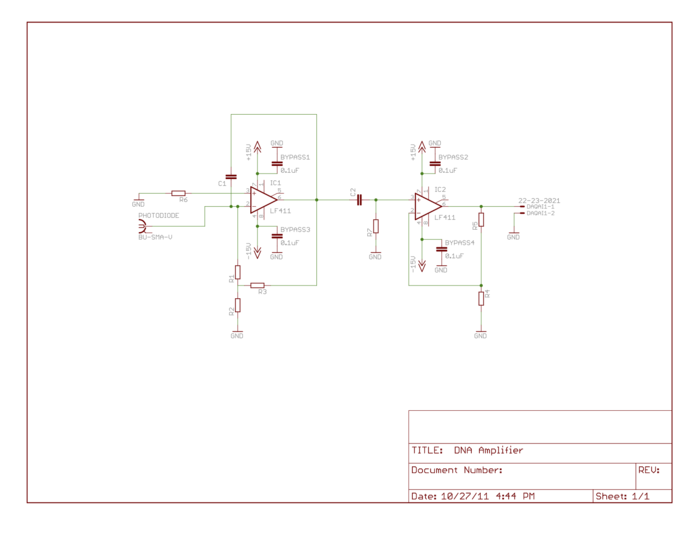
Amplifier gain adjustments and high-pass
For part 2 of the lab you will want to make some adjustments to your amplifier circuit. Principle among these are the adjustment of your low pass filter on the first stage and the addition of a high pass filter between stages. In part 1, you were amplifying an essentially constant signal, aside from it's slow variation as the dsDNA denatured. Thus your low-pass filter in stage one was most likely tuned to remove noise at 60 Hz and above. In part 2, the LED excitation light is modulated at a frequency much greater than 60 Hz and therefore the fluorescent emission from the SYBR Green is also modulated. You need to modify this low-pass filter to allow the modulated signal to get through the first stage.
Next, since the 60 Hz, 1/f, fluorescent light switching and other noise sources are likely lower than your LED modulation carrier frequency, you will want to insert a high-pass filter as shown in the updated schematic below. Think about the frequencies of the noise sources and your carrier frequency and choose an appropriate filter cutoff frequency.
Also be sure to re-evaluate the way in which you have gain distributed between the two stages. Now that the op-amps are responding to higher frequency signals, additional non-ideal characteristics may come into play. Among these is the fact that the op-amps can only slew their outputs voltages at a certain rate. Because this limited slew-rate limits the peak-to-peak voltage that can be achieved over small time scales, the maximum gain that can be achieved will likewise be limited. Review the datasheet for you LF411 op-amps, dig around online or in the course reading materials, talk to an Instructor or TA, and research two particular specifications: the slew rate and the "unity gain bandwidth" or the "gain-bandwidth product." Slew rate is directly applicable to both your first-stage transimpedence amplifier topology and your second-stage non-inverting topology. The gain-bandwidth product is an easier to use summary specification provided by the manufacturers, but it is only applicable to the op-amp's use in the typical non-inverting topolgy.
Add heater controller board
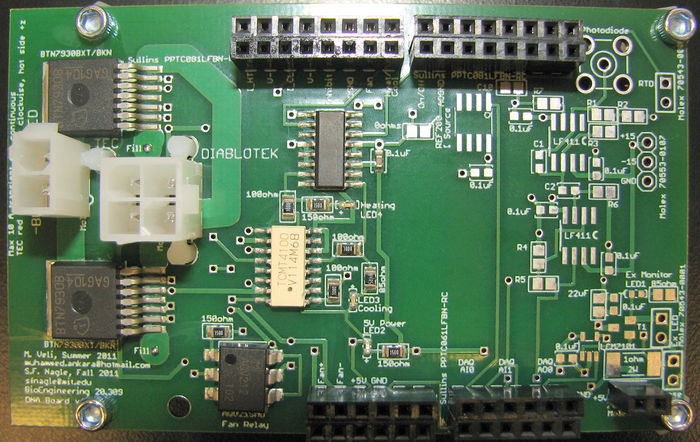
In order to control a slow ramp up and ramp down in block and sample temperature, a pulse width modulator (PWM) heater/cooler printed circuit board (PCB) has been provided for you. Once the correct connections have been made, you will be able to control your block temperature using the Labview VI with minimal input on your part. It is not required that you learn the details of the heater/cooler controller design and operation, but if you want to know more, please talk to an Instructor or TA.
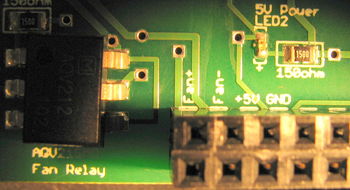
All points of connection are labeled on the PCB. Using the reference images above, make connections between your PCB and your DAQ cable (as described in the next section), your fan, and also your 5 V power. The most convenient spot from which to jump your 5 V power supply would be to tap into the location of the power connection for your blue LED. The connections to the fan itself is self-explanatory, they are labeled as "Fan+" for the red wire and "Fan-" for the black wire.
Next, connect your TEC and the computer power supply for the TEC and fan. Do not yet switch on the Diablotek computer power supply. Connect your TEC to the 2-pin Molex male connector on the left of the board using a Part 2 (red/black wires) female connector provided on the counter top near the breadboards and previously-supplied Part 1 (Black/White wires) connector. Connect these Part 2 TEC wires to your TEC stack using wire nuts as before. Be sure to connect red-to-red and black-to-black. For the "Diablotek" connector labeled on the PCB, first verify that the Diablotek computer power supply is not switched on, then use the 4-pin connector from the Diablotek that IS NOT labeled for Part 1. This will be a white connector, in contrast to the black connector that you used for Part 1.
Do not yet switch on the Diablotek.
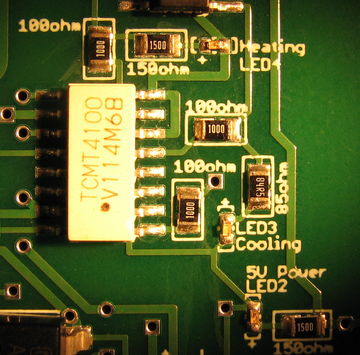
Finally, several status LEDs are provided on the PCB. A green LED will tell you whether the logic circuits are receiving 5 V power. A red LED will indicate that you are sending a heating signal. And a blue LED will indicate that you are sending a cooling signal. These LEDs do not indicate whether the TEC is actually receiving power from the Diablotek. They only indicate that status of the controller logic.
Update your PC data acquisition system
Update the DAQ connections
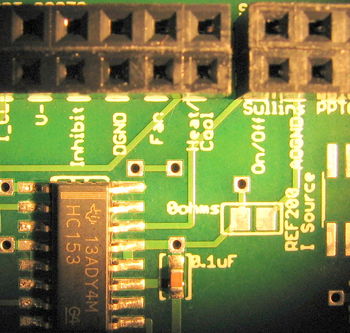
In the DAQ connections inset above, the "Fan" connection is a 0 or 5 V signal from the DAQ, controlled by the new Labview VI to be downloaded below. The "Heat/Cool" connection is a 0 or 5 V signal, also sent by the DAQ and controlled by the VI, that tells the heater/cooler logic circuits to either heat (high, 5 V) or cool (low, 0 V). The DGND slot next to both the Fan and Heat/Cool connections should be connected to the digital ground of the DAQ, which is provide using the bare wire from your DAQ connector, as described in the handout.
Next, the "On/Off" connection also accepts a 0 or 5 V signal from the DAQ. However this signal is provided a square wave of varying duty cycle. This approach forms the heart of PWM power control. When the signal is high, the power switches in the large BTN7930 half-bridge chips are configured to allow current to flow through the TEC. The Heat/Cool signal controls the direction of power flow as noted above. When the On/Off signal is low, no power flows through the switches in either direction, when it is high, power will flow. A control loop inside the Labview VI implements a Proportional-Integral-Derivative (PID) controller to set both the On/Off pulse widths and the Heat/Cool signal level in such a way as to maintain a desired temperature, or follow a desired temperature profile in time. The frequency of this duty cycle is pre-set at 750 Hz so that it's overtones do not interfere with the typical 10 kHz LED modulation frequency of your lock-in amplifier. If you choose a different LED modulation frequency, you may want to adjust the PWM frequency as well. The two half-bridge chips, connected and controlled as they are here, form what is called an H-bridge. If you want to know more, please ask an Instructor or TA.
| Signal Name | Signal Location | Ground Location | Pin wire color |
|---|---|---|---|
| DAQ Inputs | |||
| RTD | AI0+ | AI0- | +Orange / -Black |
| Photodiode | AI1+ | AI1- | +Green / -White |
| DAQ Outputs | |||
| Fan | P1.1 | N/A | Orange |
| Heat/Cool | P1.0 | N/A | Red |
| Digital GND | DGND | N/A | Black (bundled with Red/Orange) |
| Heater/Cooler On/Off | +AO1 | AOGND | +Green / -Blue or Black |
| LED Modulation Carrier | +AO0 | AOGND | +Yellow or White / -Blue |
Recall that the DAQ connections and cable wire colors are also summarized here. NOTE: the polarity of the photodiode inputs has been reversed here in order to address the cross-talk issue that we recently discovered in the DAQ.
Update and test the Labview VI
Replace your current VI with DNA_Melting_Lock-in.vi, provided for you on the course share in the Students/Labs/DNA Melting folder. Be sure to make your own copy and adjust the parameters of the RTD temperature calculations as necessary. Follow the instructions in the VI to run an experiment.
However, before you run any experiments, verify that you have connected the PCB board to the DAQ and TEC power supply appropriately. First open the VI in Labview and start it by clicking the white "run" arrow. Next turn on your +/- 15 V and 5 V power supply (the green power status LED should light on the Heater/Cooler board) and toggle the LED button to verify that your LED circuit works. If not, make a not of it to come back to it later.
Now turn on the Diablotek TEC power supply. Your fan should start at the same time. Any time the TEC power supply is on, the fan will turn on automatically when you first start-up this VI, it will turn off when you start to heat, and it will turn back on when either the temperature profile enters the cool-down phase or when you click on the cool-down override button in the VI. The fan is necessary to help your heat sink dissipate the waste heat that is pumped from the top side of the TEC stack during cooling operations.
Finally, click "Go" to start a heating cycle. The fan should turn off and the red heating status LED should light up, and the temperature of your heating block should start to respond. Be sure that your TEC power supply is on for this test. You may want to adjust the heating profile for debugging purposes.
If any of the steps above do not give the expected outcome, investigate, hypothesize, make an adjustment, and start again at the first step.
Part 2 Experimental procedure
Most of the experimental procedure for part 2 is identical to that of part 1. All of the Safety and Environmental precautions listed there also apply here. In part 2 we will give you three known dsDNA samples, and one unknown sample. The three know samples will vary in either number of base pairs in the oligos, buffer ionic strength, or the degree of match between the two oligos in solution. The unknown sample will be one of your know samples.
Your task is two-fold: 1) Produce precision melting curves for each of the three known samples and calibrate your instrument by comparing them to known standards or simulated results, and 2) produce a precision melting curve for the unknown sample and determine its identity.
To begin, open the DNA_Melting_Lock-in.vi LabView VI and follow the instructions there. Take some time to get to know your new instrument using the fluorescein and old dsDNA samples provided. Debug your instrument so that you can reliably produce a precision melting curve. Maximize SNR using the new procedure described below. Work with a TA or Instructor to verify that your instrument is in peak operating condition. Be sure that your analysis scripts are also in peak operating condition. Then and only then, work through the final experimental steps described below.
Final experiment steps
- Find your group listed on the white board in lab and retrieve your group's sample from the sample box for this semester, located in the refrigerator.
- Prepare a sample as described previously and place cuvette in your heating block.
- Use the VI as instructed there to record RTD and photodiode output during heating and cooling.
- Repeat the last steps two (2) more times for the same sample type, for a total of three (3) curves, and save the data for each run.
- Repeat the last three steps for each additional known sample type.
- Finally, prepare samples and record three (3) melting curves for your unknown sample type
| |
DO NOT leave ANY samples outside of the refrigerator unless they are in your heating block. |
Measuring signal to noise
Carry out a test of the signal to noise ratio (SNR) of your instrument by inserting a fresh sample of dsDNA and then running the VI, DNA SignalToNoise 041310.vi, provided on the course share. Run this VI with your desired operating conditions until the strip chart at the top of the screen is full of good data, and the click "Save Data" to record this data to disk. Next, open Matlab, load your file as a two column array and run it through the SToNCalculator.m function provided, using no semicolon after the function name. This function will output graphical representations of your data along with your calculated SNR numbers. Your instrument will be rated based on it's "Adjusted SNR."
Lab manual sections
- DNA Melting: Simulating DNA Melting - Basics
- DNA Melting Part 1: Measuring Temperature and Fluorescence
- DNA Melting Report Requirements for Part 1
- DNA Melting: Simulating DNA Melting - Intermediate Topics
- DNA Melting Part 2: Lock-in Amplifier and Temperature Control
- DNA Melting Report Requirements for Part 2
References
- ↑ Datasheet for the USB-6212
- ↑ Datasheet for the USB-6341
- ↑ Darlington Transistor
- ↑ TIP120 Darlington transistor
- ↑ Using a transistor
- ↑ LM741 Op-amp datasheet