Difference between revisions of "Assignment 8, Part 2: fabricate a microfluidic device"
MAXINE JONAS (Talk | contribs) (→Overview) |
Juliesutton (Talk | contribs) m (Juliesutton moved page Assignment 8, Part 1: fabricate a microfluidic device to Assignment 8, Part 2: fabricate a microfluidic device) |
||
| (22 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
==Overview== | ==Overview== | ||
| − | + | The remaining lab assignments will be based on work published in ''Science'' by MIT researchers in 2008 <ref>[http://science.sciencemag.org/content/319/5862/482 | J. T. Mettetal, D. Muzzey, C. Gomez-Uribe, and A. van Oudenaarden, "The Frequency Dependence of Osmo-Adaptation in Saccharomyces cerevisiae," Science, vol. 319, no. 5862, pp. 482–484, 2008]</ref>. The basic idea is that we will use a fluidic device to stimulate the yeast cells with high- or low-salt media, and measure the nuclear localization of the protein Hog1 using our fluorescence microscopes. We'll oscillate the flow of media from low to high salt at varying frequencies, and measure the amplitude and phase of Hog1's response. | |
| − | + | In this part of the assignment, you will make a microfluidic device that will allow you to switch the flow through a channel between two different fluid reservoirs. A previous version of the device used a method developed in Paul Blainey's lab to image the motion of transport molecules along DNA <ref>[https://www.jove.com/video/55923/a-simple-robust-high-throughput-single-molecule-flow-stretching-assay | K. Xiong and P. C. Blainey, “A Simple, Robust, and High Throughput Single Molecule Flow Stretching Assay Implementation for Studying Transport of Molecules Along DNA,” J. Vis. Exp., no. 128, pp. 1–7, 2017]</ref>. In this iteration, a channel of any shape is cut out of double-sided tape and then sandwiched between a slab of PDMS and a cover slip. In the new version of the microfluidic device, the PDMS is poured over a 3-D printed master mold and then cured. The channels are sealed with a glass coverslip. | |
| − | + | PDMS (or polydimethylsiloxane) is a silicone elastomer made by mixing together a viscous liquid base with a crossliking agent. Once mixed and annealed at 60°C, the material will harden into a solid, rubber-like material that is optically clear, non-toxic, and chemically inert. Researchers typically use PDMS for microfluidics because they can use it to cast very small sharp features (down to ~1 micron), and they can covalently bond the PDMS to a glass coverslip, creating a sealed device that is readily compatible with most types of microscopy. | |
| − | + | Typically, researchers create a master mold by etching tiny features into silicon wafers. This process involves working in a clean room and handling some nasty chemicals. Once made, the silicon waver can be used repeatedly to cast new devices out of PDMS. (The wafers are very fragile, and they typically last until they are accidentally broken!) Thankfully, since our experiment does not require extremely small features, we can get away with using a 3-D printer to fabricate our master mold. We designed a Y-shaped channel with an 800 x 800 μm cross section using CAD software, and sent the design out to a company called Protolabs for printing. | |
| + | |||
| + | ==Cast a slab of PDMS from a 3-D printed master mold== | ||
Two notes before you begin: | Two notes before you begin: | ||
| − | * This protocol has two steps with | + | * This protocol has two steps with 15-60 minute wait times. Part 2 of this assignment can be completed in parallel with Part 1, if you want to keep making progress during the downtime. |
| − | * PDMS is not harmful, but it is viscous and sticky. | + | * PDMS is not harmful, but it is viscous and sticky. Work over a sheet of aluminum foil rather than directly on the work bench, wear gloves when pouring the elastomer base and crosslinking agent, and change them before touching other lab equipment. If you spill any PDMS, clean it up right away with a paper towel or kimwipe. |
| + | * Work on a cutting mat, and not the bench, when punching holes and cutting tubing. | ||
Onward! | Onward! | ||
| − | + | [[Image: PDMSmaster.png|thumb|right|400 px|<caption>PDMS master mold.</caption>]] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ===Cast PDMS=== | |
| + | # If not already on, turn the oven on to heat to 60°C. | ||
| + | # Pour 9 g PDMS base into a plastic cup. | ||
| + | # Carefully pour 1 g of the crosslinker into the same cup, being careful to note that it is far less viscous than the base. | ||
| + | # Use a plastic stirrer to mix the crosslinker and base together really well. Stir for at least 2 minutes. | ||
| + | # Pour the mixed PDMS into a 3D printed master mold. The mold will make 3 devices. | ||
| + | # Degas the poured PDMS in the vacuum desiccator for at least 15 minutes, or until all bubbles are removed. | ||
| + | # Bake at 60°C for 1 hour. | ||
| − | === | + | ===Unmold the cured PDMS and punch inlet and outlet holes === |
| + | # Using a ceramic blade, carefully cut around the inner edge of the 3D printed mold to separate it from the PDMS. Gently pry the PDMS away from the walls with the blade and carefully peel it off of the 3D printed mold. | ||
| + | # Place the cured PDMS with the molded channels facing up on a clean cutting mat. | ||
| + | # Use the 1 mm biopsy punch to make the two inlet and one outlet holes at each end of the Y-shape. Each time you push the punch through the PDMS, make sure to remove the core. | ||
| + | # Inspect the device to ensure that each hole is cleanly formed. | ||
| + | # Store the PDMS in a clean petri dish with lid on to prevent too much dust from sticking to the surface. | ||
| + | # Repeat the punching steps for your remaining two devices. | ||
| − | + | ===Seal flow channels with a glass cover slip=== | |
| − | + | # On a clean, clutter free section of the bench, lay out a 22 x 40 mm glass coverslip and the molded PDMS device (channel side up). | |
| + | # With supervision from an instructor, turn on the corona generator and pass it back and forth over the PDMS and coverslip for about 30 s. | ||
| + | # Turn off the corona generator, then invert the PDMS onto the coverslip, trying to align the edges of the glass and PDMS as best you can. | ||
| + | # Press down firmly onto the PDMS to form a seal with the coverslip. | ||
| + | # Repeat with remaining PDMS devices. | ||
| + | # Place sealed devices in a 60°C oven for 5 minutes. | ||
| + | # If possible, leave the devices overnight before use to ensure the best seal. | ||
| − | + | ==Connect tubing== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | [[Image: ExampleTubing.jpg|thumb|right|350 px|<caption>Inlet and outlet tubing.</caption>]] | |
| − | + | Using a scalpel, cut the following lengths of tubing as marked on their packages. A summary of the tubing needed is below. Cutting the tubing on an angle will make it easier to sleeve together. | |
| + | {| class="wikitable" | ||
| + | ! style="text-align:center;"| Tubing label | ||
| + | ! Quantity | ||
| + | ! Length to cut (in inches ") | ||
| + | ! Description | ||
| + | ! Inner diameter (ID, ") | ||
| + | ! Outer diameter (OD, ") | ||
| + | |- | ||
| + | | Inlet tubing #1 | ||
| + | | 2 | ||
| + | | 11" | ||
| + | | Thin tygon tubing, semi flexible | ||
| + | | 0.02 | ||
| + | | 0.06 | ||
| + | |- | ||
| + | | Inlet tubing #2 | ||
| + | | 2 | ||
| + | | 10" | ||
| + | | Thick silicone tubing, very flexible | ||
| + | | 1/32 | ||
| + | | 3/32 | ||
| + | |- | ||
| + | | Long outlet tubing | ||
| + | | 1 | ||
| + | | 20" | ||
| + | | Thick tygon tubing, semi flexible | ||
| + | | 1/16 | ||
| + | | 1/8 | ||
| + | |- | ||
| + | | Short outlet tubing | ||
| + | | 1 | ||
| + | | 12" | ||
| + | | Thick tygon tubing, semi flexible | ||
| + | | 1/16 | ||
| + | | 1/8 | ||
| + | |} | ||
| − | + | Collect the following: | |
| + | # For each inlet, sleeve inlet tubing #1 into inlet tubing #2. Insert a luer-to-barbed fitting into the open end of inlet tubing #2. | ||
| + | # Sleeve a third luer-to-barbed fitting into one end of the long outlet tubing. | ||
| + | # Insert a bent pink needle into each of the PDMS inlet and outlet holes. | ||
| + | # Twist on the barbed fitting of the inlet tubing to the inlet needles, and set the outlet tubing aside. | ||
| − | + | Your device is now ready! Keep all your parts stored in a clean petri dish with your microscope, until you are ready to take flow data in Part 3. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
==References== | ==References== | ||
Latest revision as of 15:50, 26 March 2020
Overview
The remaining lab assignments will be based on work published in Science by MIT researchers in 2008 [1]. The basic idea is that we will use a fluidic device to stimulate the yeast cells with high- or low-salt media, and measure the nuclear localization of the protein Hog1 using our fluorescence microscopes. We'll oscillate the flow of media from low to high salt at varying frequencies, and measure the amplitude and phase of Hog1's response.
In this part of the assignment, you will make a microfluidic device that will allow you to switch the flow through a channel between two different fluid reservoirs. A previous version of the device used a method developed in Paul Blainey's lab to image the motion of transport molecules along DNA [2]. In this iteration, a channel of any shape is cut out of double-sided tape and then sandwiched between a slab of PDMS and a cover slip. In the new version of the microfluidic device, the PDMS is poured over a 3-D printed master mold and then cured. The channels are sealed with a glass coverslip.
PDMS (or polydimethylsiloxane) is a silicone elastomer made by mixing together a viscous liquid base with a crossliking agent. Once mixed and annealed at 60°C, the material will harden into a solid, rubber-like material that is optically clear, non-toxic, and chemically inert. Researchers typically use PDMS for microfluidics because they can use it to cast very small sharp features (down to ~1 micron), and they can covalently bond the PDMS to a glass coverslip, creating a sealed device that is readily compatible with most types of microscopy.
Typically, researchers create a master mold by etching tiny features into silicon wafers. This process involves working in a clean room and handling some nasty chemicals. Once made, the silicon waver can be used repeatedly to cast new devices out of PDMS. (The wafers are very fragile, and they typically last until they are accidentally broken!) Thankfully, since our experiment does not require extremely small features, we can get away with using a 3-D printer to fabricate our master mold. We designed a Y-shaped channel with an 800 x 800 μm cross section using CAD software, and sent the design out to a company called Protolabs for printing.
Cast a slab of PDMS from a 3-D printed master mold
Two notes before you begin:
- This protocol has two steps with 15-60 minute wait times. Part 2 of this assignment can be completed in parallel with Part 1, if you want to keep making progress during the downtime.
- PDMS is not harmful, but it is viscous and sticky. Work over a sheet of aluminum foil rather than directly on the work bench, wear gloves when pouring the elastomer base and crosslinking agent, and change them before touching other lab equipment. If you spill any PDMS, clean it up right away with a paper towel or kimwipe.
- Work on a cutting mat, and not the bench, when punching holes and cutting tubing.
Onward!
Cast PDMS
- If not already on, turn the oven on to heat to 60°C.
- Pour 9 g PDMS base into a plastic cup.
- Carefully pour 1 g of the crosslinker into the same cup, being careful to note that it is far less viscous than the base.
- Use a plastic stirrer to mix the crosslinker and base together really well. Stir for at least 2 minutes.
- Pour the mixed PDMS into a 3D printed master mold. The mold will make 3 devices.
- Degas the poured PDMS in the vacuum desiccator for at least 15 minutes, or until all bubbles are removed.
- Bake at 60°C for 1 hour.
Unmold the cured PDMS and punch inlet and outlet holes
- Using a ceramic blade, carefully cut around the inner edge of the 3D printed mold to separate it from the PDMS. Gently pry the PDMS away from the walls with the blade and carefully peel it off of the 3D printed mold.
- Place the cured PDMS with the molded channels facing up on a clean cutting mat.
- Use the 1 mm biopsy punch to make the two inlet and one outlet holes at each end of the Y-shape. Each time you push the punch through the PDMS, make sure to remove the core.
- Inspect the device to ensure that each hole is cleanly formed.
- Store the PDMS in a clean petri dish with lid on to prevent too much dust from sticking to the surface.
- Repeat the punching steps for your remaining two devices.
Seal flow channels with a glass cover slip
- On a clean, clutter free section of the bench, lay out a 22 x 40 mm glass coverslip and the molded PDMS device (channel side up).
- With supervision from an instructor, turn on the corona generator and pass it back and forth over the PDMS and coverslip for about 30 s.
- Turn off the corona generator, then invert the PDMS onto the coverslip, trying to align the edges of the glass and PDMS as best you can.
- Press down firmly onto the PDMS to form a seal with the coverslip.
- Repeat with remaining PDMS devices.
- Place sealed devices in a 60°C oven for 5 minutes.
- If possible, leave the devices overnight before use to ensure the best seal.
Connect tubing
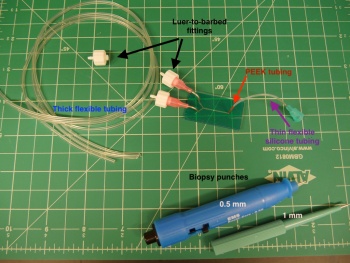
Using a scalpel, cut the following lengths of tubing as marked on their packages. A summary of the tubing needed is below. Cutting the tubing on an angle will make it easier to sleeve together.
| Tubing label | Quantity | Length to cut (in inches ") | Description | Inner diameter (ID, ") | Outer diameter (OD, ") |
|---|---|---|---|---|---|
| Inlet tubing #1 | 2 | 11" | Thin tygon tubing, semi flexible | 0.02 | 0.06 |
| Inlet tubing #2 | 2 | 10" | Thick silicone tubing, very flexible | 1/32 | 3/32 |
| Long outlet tubing | 1 | 20" | Thick tygon tubing, semi flexible | 1/16 | 1/8 |
| Short outlet tubing | 1 | 12" | Thick tygon tubing, semi flexible | 1/16 | 1/8 |
Collect the following:
- For each inlet, sleeve inlet tubing #1 into inlet tubing #2. Insert a luer-to-barbed fitting into the open end of inlet tubing #2.
- Sleeve a third luer-to-barbed fitting into one end of the long outlet tubing.
- Insert a bent pink needle into each of the PDMS inlet and outlet holes.
- Twist on the barbed fitting of the inlet tubing to the inlet needles, and set the outlet tubing aside.
Your device is now ready! Keep all your parts stored in a clean petri dish with your microscope, until you are ready to take flow data in Part 3.
References
- ↑ | J. T. Mettetal, D. Muzzey, C. Gomez-Uribe, and A. van Oudenaarden, "The Frequency Dependence of Osmo-Adaptation in Saccharomyces cerevisiae," Science, vol. 319, no. 5862, pp. 482–484, 2008
- ↑ | K. Xiong and P. C. Blainey, “A Simple, Robust, and High Throughput Single Molecule Flow Stretching Assay Implementation for Studying Transport of Molecules Along DNA,” J. Vis. Exp., no. 128, pp. 1–7, 2017
- Overview
- Part 1: feedback systems
- Part 2: fabricate a microfluidic device
- Part 3: add flow control and test your device
Back to 20.309 Main Page