Difference between revisions of "20.109(S21):M1D1"
(→Part 2: Mutagenesis of scFv clone using Error-prone PCR) |
(→Part 2: Mutagenesis of scFv clone using Error-prone PCR) |
||
| Line 86: | Line 86: | ||
|- | |- | ||
| 1 | | 1 | ||
| − | | 94 °C for 3m | + | | 94°C for 3m |
| | | | ||
| | | | ||
|- | |- | ||
| 2-11 | | 2-11 | ||
| − | | 94 °C for 45s | + | | 94°C for 45s |
| − | | 60 °C for 30s | + | | 60°C for 30s |
| − | | 72 °C for 90s | + | | 72°C for 90s |
|- | |- | ||
|12 | |12 | ||
| | | | ||
| | | | ||
| − | |72 °C for 10m | + | |72°C for 10m |
|} | |} | ||
</center> | </center> | ||
Revision as of 18:58, 27 January 2021
Contents
Introduction: Generate single-chain antibody fragment (scFv) library
To engineer a protein-protein interaction (antibody-antigen), we will use a method called directed evolution. Directed evolution is defined as an iterative process of mutating a gene, then screening those mutants for a particular functionality, collecting the best mutants and repeating. It is a method that is inspired by the process of natural selection.
In the Wittrup lab protocol, successive rounds of random mutagenesis of the scFv gene, followed by expression of the scFv on the surface of yeast then selection for "good binders" by fluorescence activated cell sorting is carried out. This process will produce an antibody fragment with increased affinity or stability to its antigen.
Today we will walk through the technical details of random DNA mutagenesis via error-prone PCR. This process creates a pool of scFv genes that have random mutations throughout the DNA sequence. This collection of varied scFV genes are called a library.
Finally, we will outline how one integrates this library into an expression vector, also referred to as the backbone or plasmid, that can then be transformed into yeast and expressed on the surface of the cells. This method is called cloning. Integral tools when cloning DNA plasmids are restriction endonucleases.
Restriction enzyme digest
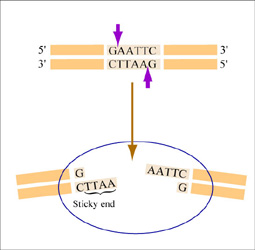
Restriction endonucleases, also called restriction enzymes, 'cut' or 'digest' DNA at specific sequences of bases. The restriction enzymes are named according to the prokaryotic organism from which they were isolated. For example, the restriction endonuclease EcoRI (pronounced “echo-are-one”) was originally isolated from E. coli giving it the “Eco” part of the name. “RI” indicates the particular version on the E. coli strain (RY13) and the fact that it was the first restriction enzyme isolated from this strain.
The sequence of DNA that is bound and cleaved by an endonuclease is called the recognition sequence or restriction site. These sequences are usually four or six base pairs long and palindromic, that is, they read the same 5’ to 3’ on the top and bottom strand of DNA. For example, the recognition sequence for EcoRI is 5’ GAATTC 3’ (see figure at right). EcoRI cleaves the phosphate backbone of DNA between the G and A of the recognition sequence, which generates overhangs or 'sticky ends' of double-stranded DNA.
Unlike EcoRI, some other restriction enzymes cut precisely in the middle of the palindromic DNA sequence, thus leaving no overhangs after digestion. The single-stranded overhangs resulting from DNA digestion by enzymes such as EcoRI are called sticky ends, while double-stranded ends resulting from digestion by enzymes such as HaeIII are called blunt ends. HaeIII recognizes 5’ GGCC 3’ and upon recognition cuts in the center of the sequence.
Protocols:
Part 1: Investigate our scFv clone
We will use SnapGene software for our DNA visualization and alignments. Feel free to use any software you are familiar with to carry out analysis. If you are off campus you must log into a VPN connection prior to opening the SnapGene application. Here is the link to the VPN download and installation instructions. You may need to update the MIT SnapGene license number if you have not opened the application recently. The new license information can be found here.
Part 2: Mutagenesis of scFv clone using Error-prone PCR
To introduce random DNA mutation into our scFv clone we will carry out error-prone polymerase chain reaction (PCR). We will use special bases in the reaction that mimic DNA oxidative damage.
| Component, concentration | Final concentration | Volume |
|---|---|---|
| 10X Taq buffer | 1X | |
| 50mM MgCl2 | 2mM | |
| 10uM Forward primer | 2mM | |
| 10uM Reverse primer | 2mM | |
| 10mM dNTPs | 200uM | |
| 100pg/uL template DNA | 13.3 pg/uL | |
| 20uM 8-oxo-dGTP | 2uM | |
| 20uM dPTP | 2uM | |
| 5 units/uL Taq polymerase | 0.05 units/uL | |
| dH2O |
Amplify DNA on the thermocycler using the following program:
| Cycles | Denaturation | Annealing | Polymerization |
|---|---|---|---|
| 1 | 94°C for 3m | ||
| 2-11 | 94°C for 45s | 60°C for 30s | 72°C for 90s |
| 12 | 72°C for 10m |
Part 3: Gel purify PCR products
Electrophoresis is a technique that separates large molecules by size using an applied electrical field and a sieving matrix. DNA, RNA and proteins are the molecules most often studied with this technique; agarose and acrylamide gels are the two most common sieves. The molecules to be separated enter the matrix through a well at one end and are pulled through the matrix when a current is applied across it. The larger molecules get entwined in the matrix and are stalled; the smaller molecules wind through the matrix more easily and travel farther away from the well. The distance a DNA fragment travels is inversely proportional to the log of its length. Over time fragments of similar length accumulate into “bands” in the gel. Higher concentrations of agarose can be used to resolve smaller DNA fragments.
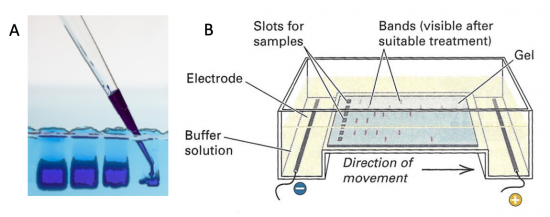
DNA and RNA are negatively charged molecules due to their phosphate backbone, and they naturally travel toward the positive electrode at the far end of the gel. Today you will separate DNA fragments using an agarose matrix. Agarose is a polymer that comes from seaweed. To prepare these gels, agarose and 1X TAE buffer (Tris base, acetic acid, and EDTA) are microwaved until the agarose is melted and fully dissolved. The molten agar is then poured into a horizontal casting tray, and a comb is added. Once the agar has solidified, the comb is removed, leaving wells into which the DNA samples can be loaded.
For the digests that were prepared in the previous laboratory session, a 1% agarose gel with SYBR Safe DNA stain was used to separate the DNA fragments in the four digest reactions. In addition, a well was loaded with a molecular weight marker (also called a DNA ladder) to determine the size of the fragments.
To ensure the steps included below are clear, please watch the video tutorial linked here: [DNA gel electrophoresis]. The steps are detailed below so you can follow along!
- Add 5 μL of 6x loading dye to the digests.
- Loading dye contains bromophenol blue as a tracking dye, which enables you to follow the progress of the electrophoresis.
- Glycerol is also included to weight the samples such that the liquid sinks into well.
- Flick the eppendorf tubes to mix the contents, then quick spin them in the microfuge to bring the contents of the tubes to the bottom.
- Load 25 μL of each digest into the gel, as well as 10 μL of 1kb DNA ladder.
- Be sure to record the order in which you load your samples!
- To load your samples
Part 4: Prepare error-prone PCR products and backbone for yeast transformation
| Component, concentration | Final concentration | Volume |
|---|---|---|
| 10X Taq buffer | 1X | 10uL |
| 50mM MgCl2 | 2mM | 4uL |
| 10uM Forward primer | 2mM | 5uL |
| 10uM Reverse primer | 2mM | 5uL |
| 10mM dNTPs | 200uM | 2.0uL |
| Gel purified PCR product | 4uL | |
| 5 units/uL Taq polymerase | 0.05 units/uL | 1uL |
| dH2O | 69uL |
| Segment | Cycles | Temperature | Time |
|---|---|---|---|
| Initial denaturation | 1 | 98 °C | 30 s |
| Amplification | 25 | 98 °C | 10 s |
| 55 °C | 30 s | ||
| 72 °C | 2 min | ||
| Final extension | 1 | 72 °C | 2 min |
| Hold | 1 | 4 °C | indefinite |
Part 5: Transform DNA into yeast
Part 6: Complete Orientation quiz
Complete the orientation quiz with your partner. Though you are working with your partner, each student should record all answers independently. If you disagree with your partner on an answer, you should write what you think is the correct answer on your quiz.
Good luck!