Difference between revisions of "20.109(S17):Complete Western blot and induce DNA damage for survival and quantitative PCR assays (Day2)"
Noreen Lyell (Talk | contribs) (→Protocols) |
Noreen Lyell (Talk | contribs) (→Introduction) |
||
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
| + | Today you will lyse the cells you seeded, isolate the protein fraction from the cell lysate, and separate the proteins on a polyacrylamide gel. You will also begin the Western blot procedure by transferring the separated proteins from your polyacrylamide gel onto a membrane. This step will enable you to 'probe' the protein fractions isolated from M059K and M059J for your protein of interest, DNA-PK. To probe the membrane, the teaching faculty will incubate it with an antibody specific to DNA-PK. This antibody is the primary antibody because it binds directly to the protein of interest. During the next laboratory session you will add a secondary antibody. The secondary antibody binds to the primary antibody and provides a means for visualization - in our experiment, the secondary antibody produces a fluorescent signal. | ||
| + | |||
| + | The ability to bind specific proteins using antibodies, or immunoglobulins, is critical in Western blot analysis. Antibodies are typically 'raised' in mammalian hosts. Most commonly mice, rabbits, and goats are used, but antibodies can also be raised in sheep, chickens, rats, and even humans. The protein used to raise an antibody is called the antigen and the portion of the antigen that is recognized by an antibody is called the epitope. Some antibodies are monoclonal, or more appropriately “monospecific,” and recognize one epitope, while other antibodies, called polyclonal antibodies, are in fact antibody pools that recognize multiple epitopes. Antibodies can be raised not only to detect specific amino acid sequences, but also post-translational modifications and/or secondary structure. Therefore, antibodies can be used to distinguish between modified (for example, phosphorylated or glycoslyated proteins) and unmodified protein. | ||
| + | |||
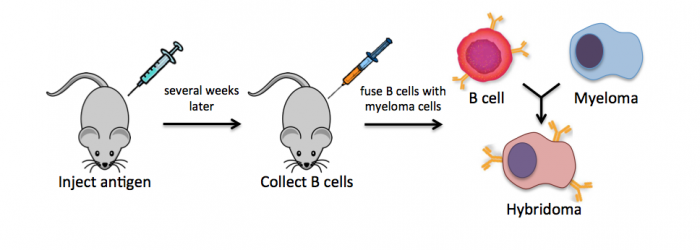
| + | Monoclonal antibodies overcome many limitations of polyclonal pools in that they are specific to a particular epitope and can be produced in unlimited quantities. However, more time is required to establish these antibody-producing cells, called hybridomas, and it is a more expensive endeavor. In this process, normal antibody-producing B cells are fused with immortalized B cells, derived from myelomas, by chemical treatment with a limited efficiency. To select only heterogeneously fused cells, the cultures are maintained in medium in which myeloma cells alone cannot survive (often HAT medium). Normal B cells will naturally die over time with no intervention, so ultimately only the fused cells, called hybridomas, remain. A fused cell with two nuclei can be resolved into a stable cell line after mitosis. | ||
| + | |||
| + | [[Image:Sp16 M2D2 monoclonal Ab.png|thumb|center|700px|'''Strategy for generating monoclonal antibodies.''']] | ||
| + | <br style="clear:both;"/> | ||
| + | |||
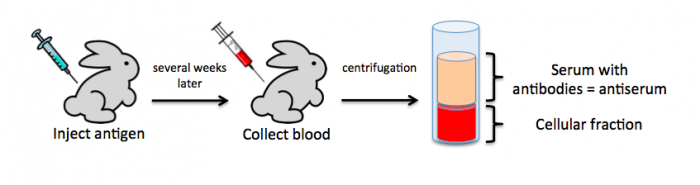
| + | To raise polyclonal antibodies, the antigen of interest is first purified and then injected into an animal. To elicit and enhance the animal’s immunogenic response, the antigen is often injected multiple times over several weeks in the presence of an immune-boosting compound called adjuvant. After some time, usually 4 to 8 weeks, samples of the animal’s blood are collected and the cellular fraction is removed by centrifugation. What is left, called the serum, can then be tested in the lab for the presence of specific antibodies. Even the very best antisera have no more than 10% of their antibodies directed against a particular antigen. The quality of any antiserum is judged by the purity (that it has few other antibodies), the specificity (that it recognizes the antigen and not other spurious proteins) and the concentration (sometimes called titer). Animals with strong responses to an antigen can be boosted with the antigen and then bled many times, so large volumes of antisera can be produced. However animals have limited life-spans and even the largest volumes of antiserum will eventually run out, requiring a new animal. The purity, specificity and titer of the new antiserum will likely differ from those of the first batch. High titer antisera against bacterial and viral proteins can be particularly precious since these antibodies are difficult to raise; most animals have seen these immunogens before and therefore don’t mount a major immune response when immunized. Antibodies against toxic proteins are also challenging to produce if they make the animals sick. | ||
| + | |||
| + | [[Image:M2D2 polyclonal antibody.png|thumb|center|700px|'''Strategy for generating polyclonal antibodies.''']] | ||
| + | <br style="clear:both;"/> | ||
| + | |||
| + | For Western blot analysis, a high quality antibody can have a relatively low affinity for its target protein. This is because the target is localized and concentrated on a blot, allowing the antibody to bind using both antibody “arms” thereby strengthening the association. Even an antibody that is loosely bound to the blot under these circumstances may dissociate then re-associate quickly since the local concentration of the target protein is high. The lower limit for protein detection is approximately 1 ng/lane, a value that varies with the size of the protein to be detected and the Western blotting apparatus that is used. For most polyacrylamide gels, the protein capacity for each lane is 100 to 200 μg (that would be 20 μL of a 5-10 μg/μL protein preparation). Thus, 1 ng represents a protein that is approximately 0.0005-0.001% of the total cellular protein (1 ng out of 100,000-200,000 ng). Proteins that make up a more significant fraction of the total protein population will be easier to detect. | ||
| + | |||
| + | In addition to the Western blot analysis preparations, you will learn more about the plasmid reporter construct you will use to measure NHEJ. As you may recall, you will use a plasmid that was engineered by the teaching faculty (thank you Leslie and Maxine!). To ensure that you are familiar with the construct and the assay details, you will think through the design considerations that went into building the NHEJ reporter. Briefly, our reporter assay works as follows: a green-fluorescent-protein-expressing plasmid is cut by a restriction enzyme(s) to produce damaged DNA, then transfected into wild-type and DNA-PK mutant cells, and repaired at some frequency that we evaluate by measuring the green fluorescence of the cell populations. | ||
==Protocols== | ==Protocols== | ||
Revision as of 19:19, 1 February 2017
Contents
Introduction
Today you will lyse the cells you seeded, isolate the protein fraction from the cell lysate, and separate the proteins on a polyacrylamide gel. You will also begin the Western blot procedure by transferring the separated proteins from your polyacrylamide gel onto a membrane. This step will enable you to 'probe' the protein fractions isolated from M059K and M059J for your protein of interest, DNA-PK. To probe the membrane, the teaching faculty will incubate it with an antibody specific to DNA-PK. This antibody is the primary antibody because it binds directly to the protein of interest. During the next laboratory session you will add a secondary antibody. The secondary antibody binds to the primary antibody and provides a means for visualization - in our experiment, the secondary antibody produces a fluorescent signal.
The ability to bind specific proteins using antibodies, or immunoglobulins, is critical in Western blot analysis. Antibodies are typically 'raised' in mammalian hosts. Most commonly mice, rabbits, and goats are used, but antibodies can also be raised in sheep, chickens, rats, and even humans. The protein used to raise an antibody is called the antigen and the portion of the antigen that is recognized by an antibody is called the epitope. Some antibodies are monoclonal, or more appropriately “monospecific,” and recognize one epitope, while other antibodies, called polyclonal antibodies, are in fact antibody pools that recognize multiple epitopes. Antibodies can be raised not only to detect specific amino acid sequences, but also post-translational modifications and/or secondary structure. Therefore, antibodies can be used to distinguish between modified (for example, phosphorylated or glycoslyated proteins) and unmodified protein.
Monoclonal antibodies overcome many limitations of polyclonal pools in that they are specific to a particular epitope and can be produced in unlimited quantities. However, more time is required to establish these antibody-producing cells, called hybridomas, and it is a more expensive endeavor. In this process, normal antibody-producing B cells are fused with immortalized B cells, derived from myelomas, by chemical treatment with a limited efficiency. To select only heterogeneously fused cells, the cultures are maintained in medium in which myeloma cells alone cannot survive (often HAT medium). Normal B cells will naturally die over time with no intervention, so ultimately only the fused cells, called hybridomas, remain. A fused cell with two nuclei can be resolved into a stable cell line after mitosis.
To raise polyclonal antibodies, the antigen of interest is first purified and then injected into an animal. To elicit and enhance the animal’s immunogenic response, the antigen is often injected multiple times over several weeks in the presence of an immune-boosting compound called adjuvant. After some time, usually 4 to 8 weeks, samples of the animal’s blood are collected and the cellular fraction is removed by centrifugation. What is left, called the serum, can then be tested in the lab for the presence of specific antibodies. Even the very best antisera have no more than 10% of their antibodies directed against a particular antigen. The quality of any antiserum is judged by the purity (that it has few other antibodies), the specificity (that it recognizes the antigen and not other spurious proteins) and the concentration (sometimes called titer). Animals with strong responses to an antigen can be boosted with the antigen and then bled many times, so large volumes of antisera can be produced. However animals have limited life-spans and even the largest volumes of antiserum will eventually run out, requiring a new animal. The purity, specificity and titer of the new antiserum will likely differ from those of the first batch. High titer antisera against bacterial and viral proteins can be particularly precious since these antibodies are difficult to raise; most animals have seen these immunogens before and therefore don’t mount a major immune response when immunized. Antibodies against toxic proteins are also challenging to produce if they make the animals sick.
For Western blot analysis, a high quality antibody can have a relatively low affinity for its target protein. This is because the target is localized and concentrated on a blot, allowing the antibody to bind using both antibody “arms” thereby strengthening the association. Even an antibody that is loosely bound to the blot under these circumstances may dissociate then re-associate quickly since the local concentration of the target protein is high. The lower limit for protein detection is approximately 1 ng/lane, a value that varies with the size of the protein to be detected and the Western blotting apparatus that is used. For most polyacrylamide gels, the protein capacity for each lane is 100 to 200 μg (that would be 20 μL of a 5-10 μg/μL protein preparation). Thus, 1 ng represents a protein that is approximately 0.0005-0.001% of the total cellular protein (1 ng out of 100,000-200,000 ng). Proteins that make up a more significant fraction of the total protein population will be easier to detect.
In addition to the Western blot analysis preparations, you will learn more about the plasmid reporter construct you will use to measure NHEJ. As you may recall, you will use a plasmid that was engineered by the teaching faculty (thank you Leslie and Maxine!). To ensure that you are familiar with the construct and the assay details, you will think through the design considerations that went into building the NHEJ reporter. Briefly, our reporter assay works as follows: a green-fluorescent-protein-expressing plasmid is cut by a restriction enzyme(s) to produce damaged DNA, then transfected into wild-type and DNA-PK mutant cells, and repaired at some frequency that we evaluate by measuring the green fluorescence of the cell populations.
Protocols
Part 1: Induce DNA damage
Part 1a: Treat cells for survival assay
Part 1b: Treat cells for quantitative PCR assay
Part 2: Complete Western blot
Part 3: Design primers for quantitative PCR assay
Part 4: Discuss research paper
We will end today with a discussion of the Dietlein et al. research article. In their research, the authors completed a screen to examine 1319 cancer-associated genes from 67 cell lines to identify cancer-cell specific mutations that are associated with DNA-PKcs dependence or addiction.
Our paper discussion will assist you in writing a cohesive story that clearly reports the data and provides strong support for the conclusions made about the data. During the paper discussion, everyone is expected to participate - either by volunteering or by being called upon!
Introduction
Remember the key components of an introduction:
- What is the big picture?
- Is the importance of this research clear?
- Are you provided with the information you need to understand the research?
- Do the authors include a preview of the key results?
Results
Carefully examine the figures. First, read the captions and use the information to 'interpret' the data presented within the image. Second, read the text within the results section that describes the figure.
- Do you agree with the conclusion(s) reached by the authors?
- What controls are included and are they appropriate for the experiment performed?
- Are you convinced that the data are accurate and/or representative?
Discussion
Consider the following components of a discussion:
- Are the results summarized?
- Did the authors 'tie' the data together into a cohesive and well-interpreted story?
- Do the authors overreach when interpreting the data?
- Are the data linked back to the big picture from the introduction?
Reagents
Next day: Assess cell survival and harvest RNA for quantitative PCR assay
Previous day: Confirm cell lines and practice tissue culture techniques