Difference between revisions of "20.109(S16):Begin Western blot protein analysis and choose system conditions (Day2)"
Noreen Lyell (Talk | contribs) (→Part 4: Transferring proteins onto a membrane) |
Noreen Lyell (Talk | contribs) (→Part 4: Transferring proteins onto a membrane) |
||
| Line 149: | Line 149: | ||
#After the transfer is complete, turn off the current, disconnect the blotting tank from the power supply, and remove the transfer cassettes. | #After the transfer is complete, turn off the current, disconnect the blotting tank from the power supply, and remove the transfer cassettes. | ||
#Retrieve the nitrocellulose filter and confirm that the pre-stained standard markers transferred from the gel to the membrane. | #Retrieve the nitrocellulose filter and confirm that the pre-stained standard markers transferred from the gel to the membrane. | ||
| − | #Cut the membrane such that each team has a membrane with only their samples, and then transfer | + | #Cut the membrane such that each team has a membrane with only their samples, and then transfer each membrane to a plastic dish with blocking buffer. |
#Your membranes will be stored at 4°C. | #Your membranes will be stored at 4°C. | ||
Revision as of 22:56, 5 February 2016
Contents
- 1 Introduction
- 2 Protocols
- 3 Reagent list
- 4 Navigation links
Introduction
Previously you learned more about the two cell lines that we will be using during Module 2, and seeded a known quantity of each cell type in preparation for protein analysis. The protein analysis that you will complete serves two important research purposes: 1. The DNA-PK mutant is a negative control and 2. When compared to the wild type, the mutant may give insight into the role of DNA-PK in NHEJ dsb repair.
Today you will lyse the cells you seeded, isolate the protein fraction from the cell lysate, and separate the proteins on a polyacrylamide gel. You will also begin the Western blot procedure by transferring the separated proteins from your polyacrylamide gel onto a membrane. This step will enable you to 'probe' the protein fractions isolated from M059K and M059J for your protein of interest, DNA-PK. To probe the membrane, the teaching faculty will incubate it with an antibody specific to DNA-PK. This antibody is the primary antibody because it binds directly to the protein of interest. During the next laboratory session, you will add a secondary antibody. The secondary antibody binds to the primary antibody and provides a means for visualization - in our experiment, the secondary antibody produces a fluorescent signal.
The ability to bind specific proteins using antibodies, or immunoglobulins, is critical in Western blot analysis. Antibodies are typically 'raised' in mammalian hosts. Most commonly mice, rabbits, and goats are used, but antibodies can also be raised in sheep, chickens, rats and even humans. The protein used to raise an antibody is called the antigen and the portion of the antigen that is recognized by an antibody is called the epitope. Some antibodies are monoclonal, or more appropriately “monospecific,” and recognize one epitope, while other antibodies, called polyclonal antibodies, are in fact antibody pools that recognize multiple epitopes. Antibodies can be raised not only to detect specific amino acid sequences, but also post-translational modifications and/or secondary structure. Therefore, antibodies can be used to distinguish between modified (for example, phosphorylated or glycoslyated proteins) and unmodified protein.
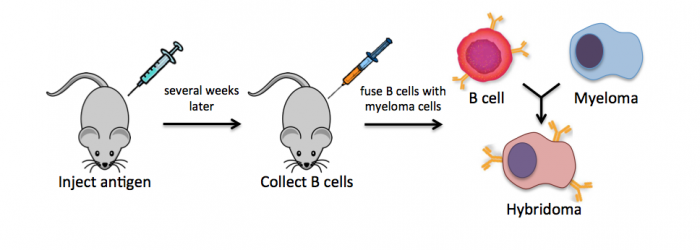
Monoclonal antibodies overcome many limitations of polyclonal pools in that they are specific to a particular epitope and can be produced in unlimited quantities. However, more time is required to establish these antibody-producing cells, called hybridomas, and it is a more expensive endeavor. In this process, normal antibody-producing B cells are fused with immortalized B cells, derived from myelomas, by chemical treatment with a limited efficiency. To select only heterogeneously fused cells, the cultures are maintained in medium in which myeloma cells alone cannot survive (often HAT medium). Normal B cells will naturally die over time with no intervention, so ultimately only the fused cells, called hybridomas, remain. A fused cell with two nuclei can be resolved into a stable cell line after mitosis.
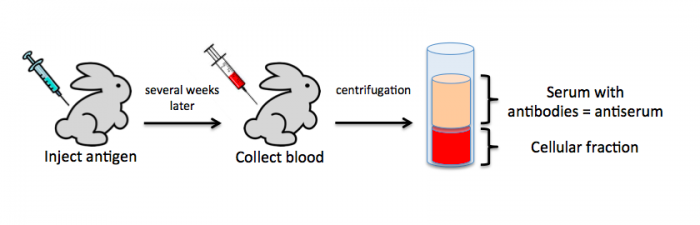
To raise polyclonal antibodies, the antigen of interest is first purified and then injected into an animal. To elicit and enhance the animal’s immunogenic response, the antigen is often injected multiple times over several weeks in the presence of an immune-boosting compound called adjuvant. After some time, usually 4 to 8 weeks, samples of the animal’s blood are collected and the cellular fraction is removed by centrifugation. What is left, called the serum, can then be tested in the lab for the presence of specific antibodies. Even the very best antisera have no more than 10% of their antibodies directed against a particular antigen. The quality of any antiserum is judged by the purity (that it has few other antibodies), the specificity (that it recognizes the antigen and not other spurious proteins) and the concentration (sometimes called titer). Animals with strong responses to an antigen can be boosted with the antigen and then bled many times, so large volumes of antisera can be produced. However animals have limited life-spans and even the largest volumes of antiserum will eventually run out, requiring a new animal. The purity, specificity and titer of the new antiserum will likely differ from that of the first batch. High titer antisera against bacterial and viral proteins can be particularly precious since these antibodies are difficult to raise; most animals have seen these immunogens before and therefore don’t mount a major immune response when immunized. Antibodies against toxic proteins are also challenging to produce if they make the animals sick.
For Western analysis, a high quality antibody can have a relatively low affinity for its target protein. This is because the target is localized and concentrated on a blot, allowing the antibody to bind using both antibody “arms” thereby strengthening the association. Even an antibody that is loosely bound to the blot under these circumstances may dissociate then re-associate quickly since the local concentration of the target protein is high. The lower limit for protein detection is approximately 1 ng/lane, a value that varies with the size of the protein to be detected and the Western blotting apparatus that is used. For most polyacrylamide gels, the protein capacity for each lane is 100 to 200 μg (that would be 20 μL of a 5-10 μg/μL protein preparation). Thus, 1 ng represents a protein that is approximately 0.001-0.002% of the total cellular protein (1 ng out of 100,000-200,000 ng). Proteins that make up a more significant fraction of the total protein population will be easier to detect.
In addition to the Western blot analysis preparations, you will learn more about the plasmid reporter construct you will use to measure NHEJ. As you may recall, you will use a plasmid that was engineered by the teaching faculty (thank you Leslie and Maxine!). To ensure that you are familiar with the construct and the assay details, you will think through the design considerations that went into building the NHEJ reporter. Briefly, our reporter assay works as follows: a green-fluorescent-protein-expressing plasmid is cut by a restriction enzyme(s) to produce damaged DNA, then transfected into wild-type and DNA-PK mutant cells, and repaired at some frequency that we evaluate by measuring the green fluorescence of the cell populations.
Protocols
Part 1: Preparing cell lysates
In this exercise you will prepare your samples for Western blot analysis. It is very important that you keep the cell lysate cold throughout the procedure.
- You have an ice bucket at your bench with the following pre-chilled items inside: two empty eppendorfs, RIPA buffer, protease inhibitors, and PBS.
- Label the eppendorf tubes as M059K and M059J (plus your section).
- Retrieve your 6-well plate from the incubator in TC, and hold it at a 30-45° angle in your bucket.
- You may find it helpful to push the ice such that you build a ramp in the bucket that you can use to hold the plate steady.
- Add 10 μL of protease inhibitors to your 1000 μL of aliquotted RIPA buffer and return this mixture to the ice.
- Aspirate the media from each well by holding the tip of the pasteur pipet (be sure to cap the pasteur pipet with a yellow tip!) at the bottom of the well.
- Add 2 mL of ice-cold PBS to each well.
- Obtain two pre-chilled scrapers from the 4 °C cooler.
- Aspirate the ice-cold PBS and repeat the wash once more – be sure to remove ALL of the PBS after this wash.
- Add 100 μL of lysis buffer to the top of each well and allow it to run down the well to the bottom.
- Dislodge the cells from the well by scraping each well with a fresh, cold cell scraper.
- First tilt the plate back and forth to coat the cells with lysis buffer.
- Then go from top to bottom 'windshield wiper style' to pool the cells at the bottom of each well in the tilted plate.
- Transfer the cells from each well to the appropriate eppendorf tube.
- At this step it is important to make sure there are no aggregates or clumps of cell lysate remaining in the wells. Do this by tilting the plate so that light reflects off the bottom. If you see any 'chunks', pipette your lysate back into the well to dissolve the chunk, then re-transfer everything into the eppendorf tube.
- Incubate the eppendorf tubes with your cell lysates on ice for 10 min.
- Meanwhile, label two fresh eppendorf tubes and add them to your ice bucket to chill for a later step.
- Spin the cell lysate at maximum speed in the cold room centrifuge for 10 min to pellet insoluble material.
- The teaching faculty will show take you to the centrifuge when you are ready.
- This step is typically referred to as "clearing" the lysate. The pellet at the bottom contains the DNA from the cell and genomic DNA can get very soupy making it difficult to load your lysate on the SDS-PAGE gel.
- Transfer the supernatant to the fresh eppendorf tubes – be careful not to disturb the pellet at the bottom!
- Keep your samples on ice when not in use.
Part 2: Measuring protein concentration
You will now measure the total protein concentration in each cell lysate to determine the volume that you will use for the polyacrylamide gel separation.
- Immediately before it is your turn to use the spectrophotometer, add 10 μL of each cell lysate to a plastic cuvette.
- Prepare a 'blank' by adding 10μL of your leftover RIPA buffer to a cuvette.
- Be careful not to allow 10 μL of lysate to sit in the cuvettes for more than a couple minutes before starting the next step -- the liquid will evaporate!
- Carefully take your cuvettes to the spectrophotometer and add 990 μL of Precision Red reagent.
- Mix by pipetting up and down a 2-3x without introducing bubbles.
- Wait 1 minute, then measure each sample at 600 nm.
- Use the RIPA sample to blank the spectrophotometer.
- Calculate the two stock protein concentrations of your M059K and M059J cell lysate using the following information:
- 1 absorbance unit = 100 ug protein/mL reagent / cm
- The path length of the cuvette is precisely 1 cm.
- Remember to account for the dilution factor!
- Next, calculate the volumes of lysate and water required to add 10 - 20 μg of total protein to the polyacrylamide gel in a total volume of 20 μL, per lysate sample.
- If your concentration is greater than 1μg/μL, use water to make up the remaining volume.
- If your concentration is less than 1μg/μL for at least one sample, scale both samples down to a lower protein amount, such as 10 μg
- Do not throw away the remainder of your cell lysate! We will store these samples at -80 °C in case your Western blot needs to be repeated.
Part 3: Separating proteins using polyacrylamide gel electrophoresis (PAGE)
You will now prepare your cell lysate samples for separation using PAGE. Before you 'load' your samples into the gel the teaching faculty will demo proper technique so you do not lose your sample in the buffer or break the gel cartridge. Two teams will share one gel. Because of the limited space in the gel electrophoresis area of the laboratory, please wait for your turn to load your samples at your bench.
- Working inside the chemical fume hood, add 4 μL of 6X Laemlli sample buffer to both of your cell lysates. Be sure to wear your safety goggles at the hood!
- Eject your β-mercaptoethanol-contaminated tips into the empty pipette tip box inside the hood so they can air out before disposing of them in the biohazard waste box. Fun fact: humans can smell this stinky compound at the order of one part per million!
- Briefly vortex each tube, then centrifuge to collect the samples at the bottom of the tubes.
- Obtain 10μL of the Dual Color protein molecular weight standard ladder and put it in a fresh, labeled eppendorf tube.
- Put lid locks on the eppendorf tubes – including the ladder – and boil for 5 minutes in the water bath that is in the fume hood.
- Repeat Step #2.
- When your samples are ready, alert the teaching faculty so they can show you how to properly load the gel.
- It is okay to let your samples boil 2-3 extra minutes if there is not a free gel box, but don't boil for more than 10 min total.
- It is also okay to simply remove them from the water bath and let them cool for a couple of minutes.
- Load your samples according to the scheme below.
- In your notebook, document the start and stop time of electrophoresis.
- The teaching faculty will begin electrophoresis after both groups load their samples.
- The proteins will be separated using 200 V for 30-40 minutes.
- During electrophoresis, work through Part 5 with your laboratory partner.
| Lane | Sample | Volume to load |
|---|---|---|
| 1 | Team 1, M059K | 20 μL |
| 2 | Team 1, M059J | 20 μL |
| 3 | "Dual Color" protein molecular weight standards | 2 μL |
| 4 through 6 | EMPTY | N/A |
| 7 | Team 2, M059K | 20 μL |
| 8 | Team 2, M059J | 20 μL |
| 9 | "Dual Color" protein molecular weight standards | 2 μL |
| 10 | EMPTY | N/A |
Part 4: Transferring proteins onto a membrane
After the electrophoresis procedure, the teaching faculty will assist you in assembling the transfer cassette according the below protocol. If we run short on time, the teaching faculty will complete this part for you.
- Wearing gloves, carefully disassemble the electrophoresis chamber.
- Assemble the transfer cassette in the following order:
- Place the grey side of the transfer cassette in a tupperware container with transfer buffer. The transfer cassette is color-coded so the grey side should face the cathode (black electrode) and the clear side should face the anode (red electrode).
- Place a ScotchBrite pad pre-soaked in transfer buffer on the black side of the cassette.
- Place 1 piece of filter paper on top of the ScotchBrite pad.
- Place your gel on top of the filter paper.
- Lightly press out any air bubbles that form between the filter paper and your gel.
- Place a piece of nitrocellulose membrane on top of the gel.
- The nitrocellulose membrane is white and should be kept between the blue protective paper sheets until use. Wear gloves when handling the membrane to avoid transferring proteins from your fingers to the filter.
- Again, lightly press out any air bubbles that form between the gel and the nitrocellulose membrane.
- Place another piece of filter paper on top of the nitrocellulose.
- Place a second ScotchBrite pad pre-soaked in transfer buffer on top of the filter paper.
- Close the cassette, then push the clasp down and slide it along the top to hold it together.
- Place the transfer cassette into the blotting tank so that the clear side faces the red electrode and the grey side faces the black electrode.
- Two blots can be run in each tank. When both are in place, insert an ice compartment into the tank and fill the tank with transfer buffer. Connect the power supply and transfer at 100 V for 60 min.
- After the transfer is complete, turn off the current, disconnect the blotting tank from the power supply, and remove the transfer cassettes.
- Retrieve the nitrocellulose filter and confirm that the pre-stained standard markers transferred from the gel to the membrane.
- Cut the membrane such that each team has a membrane with only their samples, and then transfer each membrane to a plastic dish with blocking buffer.
- Your membranes will be stored at 4°C.
Part 5: Reverse engineering pMax-BFP-MCS
Understanding existing pMax-BFP plasmid
- Begin by downloading the pMax-BFP file and opening it in ApE. This plasmid is a recent iteration of the BFP component in the NHEJ assay developed in Samson lab.
- Also open the product page for the original pMax cloning vector from Lonza, found here.
- You will use the above two resources to answer several questions below.
- What three components are related to propagating the plasmid in bacteria? Why is propagation in bacteria useful?
- What three components are related to plasmid expression in mammalian cells? What is the purpose of each?
- Look at the product page MCS, and compare it to the MCS that you view in ApE. How do they differ? Let's break this question down further.
- What restriction site(s) have been deleted? (See hint below!)
- What restriction site(s) have been added? (Ditto.)
- Hint: choose "Graphic Map + U" under the Enzymes file menu to see single-cutters. Use base-pair numbers and feature labels, along with the Lonza MCS sequence, to orient yourself.
- Recall that mammalian cells require a Kozak sequence for translation. Can you find one here?
- So, by now you should see that much of the original MCS has been deleted.
- This plasmid was prepared from pMax in two steps. First, the MCS deletion was done. Next, the insertion of BFP and an additional restriction site was done. Of the sites remaining in the MCS, which appear to have been used to clone in BFP?
- What's special about the cut topology of the newly introduced restriction site that is present in the BFP insert? How does it differ from those remaining in the truncated pMax MCS?
- To answer this question, you will need to look up the individual restriction enzymes on the New England Biolabs website. For example, the page for KpnI is found here
- Note that NEB indicates cut sites for the restriction recognition sequence with small triangles.
(Re)-designing pMax-BFP-MCS plasmid
By now you should see that pMax-BFP has a restriction site that produces blunt ends upon cutting. With respect to assay ease of use, blunt ends are convenient because the associated DNA requires minimal or even no purification after digestion. In contrast, after digestion that produces cohesive ends, it is wise to get rid of the tiny fragments, lest they fill back in on the plasmid and change re-ligation efficiency.
For our study, however, we are interested in comparing many different kinds of digested ends. Given that we are performing extra steps anyway, wouldn't it be nice to be confident that we successfully cut our DNA? Based on techniques you learned in Module 1, think about how you might accomplish this validation. We'll come to the answer a little later.
In order to create various digested ends, we will design an extended MCS. It is important that the restriction enzymes that we pick don't occur somewhere else in pMax or in the BFP code-determining sequence. Why? To find these restriction enzymes, we will use another tool from NEB, namely NEBcutter.
- Copy the pMax-BFP sequence into the query box. You can keep most of the default parameters, but one should stand out to you as "wrong." Adjust it, name your project, and then submit the sequence.
- View the "0 cutter" enzymes, i.e., the enzymes that don't cut this plasmid. How many restriction sites (of those recognized by NEB-available enzymes) are absent from pMax-BFP? How many of these are cut in a blunt fashion?
- Assuming that you will need about ten restriction sites in your MCS, how would you possibly go about narrowing this zero-cutter list down? Brainstorm a few ideas with your partner before reading ahead.
- In fact, the following considerations entered into our design decisions
- enzymes that are all or mostly all compatible in the same buffer
- enzymes that are relatively common (i.e., have uses in other lab modules)
- enzymes that we already had in the lab
- enzymes that avoid long stretches of AT or GC
- enzymes that don't require many base-pairs on either side of the recognition site for successful cutting see here
We eventually jettisoned the last consideration, when in a planning meeting we came up with an idea for validating DNA cutting. Did you think of any ideas yet? Take a moment to brainstorm… We decided that instead of making one MCS, we would make two, and that these would be separated by a nonsense DNA fragment large enough to see on a gel. For example, we might cut with a sticky enzyme in the left-hand MCS (hereafter MCS1), and a blunt enzyme in the right-hand MCS (hereafter MCS2), creating a hybrid break site. Afterward, the DNA could be run on a gel, both to validate cutting (by observing release of the nonsense fragment), and to isolate/purify the cut plasmid reporter. But how should we deal with a more simple cut topology, such as sticky alone or blunt alone? Aha! The same restriction site should be present in both MCS1 and MCS2.
- With the above design strategy in mind, download the MCS file and open it in ApE.
- The very end of each MCS (5' of MCS1 and 3' of MCS2) was used to clone the dual-MCS construct into pMax-BFP. That is, the MCS file represents the insert and the pMax-BFP file represents the vector.
- What enzyme recognizes this end sequence that was used for cloning?
- Hint: just try Googling the first 6 bp of the MCS.
- Given the information you encountered in the design considerations above, what do you think the purpose of the "cut cap" is?
- What enzyme recognizes this end sequence that was used for cloning?
- Manually "clone" the MCS construct into the pMax-BFP file, and save it with a new name, such as pMax-BFP-MCS. What is the total size of the new plasmid?
- With your mouse/trackpad, highlight from the end of MCS1 to the end of MCS2, encompassing the whole insert. Under the Enzymes file menu, choose Selection Only.
- Next, go to Enzymes → Enzyme Selector. Choose "unique" from the lower drop-down menu, and press "Select." Next, choose "equal to 2" and again press "Select." Finally, press "Graphic Map" (not "Graphic Map + U," as "U" will override your previous selections and show only unique enzymes).
- If you wish to, print this graphic for your reference. Note that because of the handy menus in ApE, we are viewing only the insert part of pMax-BFP-MCS, but we are being told (in parentheses) the number of restriction sites for a given enzyme that exist in the entire plasmid. Otherwise, you would need to cross-check against the zero-cutters list to cross these out.
- Paying attention only to the true single- and dual-site enzymes, design a digest to prepare each of the following topologies, using a single enzyme or pair of enzymes for each:
- sticky ends, 5' overhang
- sticky ends, 3' overhang
- blunt ends
- 5' sticky end on upstream side, blunt end on downstream side
- 3' sticky end on upstream side, blunt end on downstream side
- sticky ends that are topologically compatible, but that have one or more sequence mismatches
- sticky ends that are topologically incompatible (overhang is on top strand for both, for example).
- For the double digests, try to choose enzymes that are compatible with the same buffer. The NEB buffer chart can be found linked here. Sometimes using high fidelity (HF) enzymes will be a better bet than the originals, and sometimes a worse bet.
Reagent list
From Boston Bioproducts unless otherwise noted:
- RIPA Lysis Buffer
- 50 mM Tris-HCL, pH 7.4
- 150 mM NaCL
- 1% NP-40
- 0.5% Sodium deoxycholate
- 0.1% SDS
- 100X Protease Inhibitor cocktail
- AEBSF
- Aprotinin
- E-64 Besstain Leupeptin
- EDTA
- Precision Red Advanced Protein Assay (Cytoskeleton, Inc.)
- 6x Reducing Laemlli Sample Buffer
- 375 mM Tris HCL, pH 6.8
- 9% SDS
- 50% Glycerol
- 9% Betamercaptoethanol
- 0.03% Bromophenol blue
From Bio-Rad
- 4-20% Mini-PROTEAN TGX gel
- TGS Buffer: 25 mM Tris, 192 mM glycine, 0.1% (w/v) SDS, pH 8.3
- Dual Color marker sizes here
- Transfer Buffer
- 25 mM Tris
- 192 mM Glycine
- 20% v/v Methanol
Next day: Complete Western and prepare damaged DNA
Previous day: Introduction to cell strains and plating