Procedure: Diffusion in biological gels
You are going to work with sensitive biological samples, so wear gloves at all times when you prepare the sample to avoid contamination!
Part I: finding a suitable particle dilution
You are going to test the diffusion behavior of three different particle types in matrigel. The three particle species are:
a) 1 µm liposomes, positively charged
b) 1 µm liposomes, neutral
c) 100 nm polystyrene beads, positively charged
You first have to determine a suitable particle concentration for your tracking experiment. You want to have more than 1 particle per ROI for tracking but not too many particles (since then the contrast will suffer). Since the particle concentration in the stock solutions you are given is quite high, you have to find an appropriate dilution for each particle type. All particles are labeled with a red fluorophore.
1) Prepare a dilution of your stock solution (10-50 µl will do). For better comparability to later experiments, add 1 µl of your particle dilution to 50 µl of water to create a ”sample”.
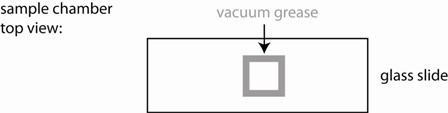
2) Use the vacuum grease filled syringe to place an open ~ 15x15 mm square on the middle of a glass slide. Make sure it is small enough so that it can be fully covered with a cover slip. Ensure that the linings are continuous (see figure), otherwise your sample chamber will leak and you will have lots of drift.
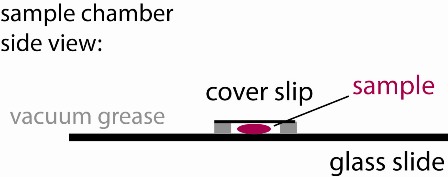
3) Place ~30 µl of your first sample into the vacuum grease well of the sample chamber you have crafted in step 2. Cover it with a cover slip creating a sandwich (see figure). Label the glass slide to keep track of your sample.
Repeat steps 1-3 until you have found suitable dilutions for all three particles.