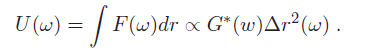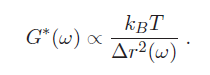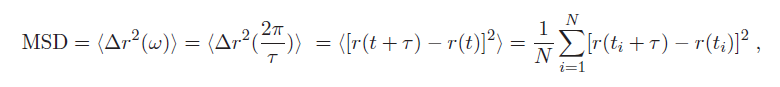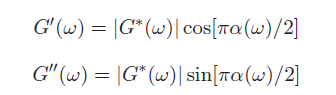Optical Microscopy Part 4: Particle Tracking
Introduction and Background
Microrheology
Many cellular functions such as migration, differentiation, and proliferation are regulated by the mechanical properties of cells, specifically, their elasticity and viscosity. Rheology is the science of measuring materials' mechanical properties. Microrheology is a subgroup of techniques that are capable of measuring mechanical properties from microscopic material volumes. Clearly, given the typical size of biological cells, microrheology is the technique needed to measure their elasticity and viscosity. The elastic and viscous properties of cells can be characterized by a complex-valued shear modulus (with units of Pa) G*(ω) = G'(ω) + iG"(ω). The real part G'(ω), referred to as the storage modulus, is a measure of cell elasticity, while the imaginary part G"(ω), the loss modulus, is a measure of their viscosity. A generalized Hookean relationship can be written as:
where Δr is a generalized displacement, and F(ω) is a force linearly proportional to it via the shear modulus. Therefore, we can measure the shear modulus if we can measure the deformation of the cell under a known force. (Note that all these quantities are frequency-dependent). Particle-tracking microrheometry is based on measuring the displacement of a particle with radius $ a $ embedded in a cell driven by thermal forces. One complication is that this relationship is frequency dependent - this is because in complex fluids, such as the cellular cytoskeleton, there are different energy dissipation mechanisms over different time scales. To approach the derivation of the relevant formulas, it is more convenient to think in terms of energy, rather than force. The relationship between stored energy and displacement has a familiar form, similar to a spring-mass system (recall $ KE \ \alpha \ k(\omega)^2 $ ):
What is the driving thermal energy U(ω)? Recall also that thermal energy is "white," i.e. it contains equal power at all frequencies and is equal to 0.5*k$ _B $T for each degree of freedom in a second-order system, where k$ _B $ is Boltzmann's constant and T is the absolute temperature. From this relationship (since we're observing motion in two dimensions), we have
Our argument is obviously very approximate. A complete (and much more difficult) derivation results in the following equation (see Mason [1] for details):
$ \left\vert G^*(\omega) \right\vert = {k_B T \over \pi a \left \langle \Delta r^2 (\omega) \right \rangle \Gamma[1 + \alpha(\omega)]} $
Some key additional details to help you make sense of this equation:
1. As you can see, the dependence on displacement is more accurately expressed as the mean squared displacement (MSD) <Δr$ ^2 $(ω)>:
where < > denotes a time-average of the particle's displacement trajectory r(t), at discrete times t = t$ _1 $,.... t$ _n $ (as sampled by a digital system like the PC and camera). Additionally, τ is a characteristic lag/delay time for the measurement. corresponding to the frequency ω.
2. $ \alpha(\omega)=\frac{\partial \ln \left \langle \Delta r^2(\tau) \right \rangle }{\partial \ln \tau} $
3. The radius of the particle $ a $ plays a role in the formula.
4. Γ(.) is the Gamma function (the generalized form of the factorial function, which can be looked up in a mathematical table). Mason suggests that for our range of α,
$ \Gamma[1 + \alpha] \approx = 0.457(1 + \alpha)^2 - 1.36(1 + \alpha) + 1.90. $
This equation may look complicated but there is a simple approximation to calculate the elastic and viscous moduli:
A detailed discussion of particle tracking microrheology can be found in the papers by Mason and Lau[2] An application of the work of Mason et al to 3T3 cells in particular can be found in the work of Tseng et al. [3]
Cell culture and labeling protocols
Read Optical Microscopy Part 3c: Protocols for cell culture, cell fixing and cell imaging.
Effects of cytochalasin D on actin cytoskeleton
This last part of the lab module seeks to illustrate the effect of cytochalasin D (CytoD) on cells. Since there are significant rheological changes in 3T3 fibroblasts treated with CytoD, it is reasonable to assume that this chemical may modify the cells' cytoskeleton. We will image actin (one of the most important component of the mammalian cell cytoskeleton) structures in these cells, with and without CytoD treatment.
The actin cytoskeleton is visualized using the toxin phalloidin labeled with Alexa Fluor 568 (excitation maximum: 578nm, and emission maximum: 600nm). Phalloidin is a fungal toxin (small organic molecule that hinders actin assembly) that binds only to polymerized filamentous actin (F-actin), but not to actin monomers, G-actin.
Instructions
Actin imaging
| |
Wear gloves when you are handling biological samples. |
Since actin filaments and stress fibers are nanometer-scale objects, they are much dimmer than fluorescent
beads or the dye solution - care must be taken to get good images of the cytoskeleton. You may
need to cover the microscope to reduce room light contamination.
- Adjust the gain and exposure of the camera to get the best picture. Be sure to keep the same exposure conditions, however, for both untreated and CytoD-treated cells.
- Using the 40x objective, take some good fluorescence images of Alexa Fluor phalloidin-labeled cells, both untreated and CytoD-treated.
Live cell microrheology by particle traking
Now that you have verified microscope stability and performance through the particle tracking lab, you can apply its methods on cell samples. A key technique to keep in mind when working with live cells - to avoid shocking them with "cold" at 20°C, be sure that any solutions you add are pre-warmed to 37°C. We will keep a warm-water bath running for this purpose, in which we will keep the various media.
You are provided with NIH 3T3 fibroblasts, which were prepared as follows: Cells were cultured at 37°C in 5% CO$ _2 $ in standard T75 flasks in a medium referred to as DMEM++. This consists of Dulbecco's Modified Eagle Medium (DMEM - Invitrogen) supplemented with 10% fetal bovine serum (FBS - Invitrogen) and 1% of the antibiotic penicillin-streptomycin (Invitrogen). The day prior to the microrheology experiments, fibroblasts were plated on 35 mm glass-bottom cell culture dishes (MatTek). On the day of the experiments, the cell confluency should reach about 60%. 1 μm diameter orange fluorescent microspheres (Molecular Probes) were mixed with the growth medium (at a concentration of 5 x 10$ ^5 $ beads/mL) and added to the plated cells for a period of 12 to 24 hours for bead endocytosis.
- Carefully pipet out the medium from both dishes. Replace with 2 mL of fresh, pre-warmed DMEM++ in each dish.
- Choose cells with 3 or 4 particles embedded in them and capture movies of the samples. Take multiple movies with about 3-5 cells in the field of view in each movie.
- In one of the dishes, treat the cell with the cytoskeleton-modifying chemical cytochalasin D (CytoD): Pipet out the medium, add 1 mL CytoD solution at 10 μM (pre-mixed for you) to the dish, and wait for 20-30 minutes. It's a good idea to check on your cells after 20 minutes: sometimes they are in bad shape at that point but sometimes they still look very healthy. Wash 2X with 2 mL of pre-warmed DMEM++, leaving 2 mL in the dish when imaging.
- Perform and repeat the particle tracking measurements again as quickly as you are able. The cells' physiology has now been significantly disrupted by the toxin CytoD, and they will die within a couple of hours. It's very unlikely that you'll be able to find the exact same cells you've already tracked; however it's very much advisable to use the same dish for the "before" and "after" so you're aren't also comparing between different cell populations.
Lab manual homepage
Lab Manual: Optical Microscopy
References
- ↑ T. G. Mason, "Estimating the viscoelastic moduli of complex fluids using the generalized Stokes-Einstein equation" Rheol. Acta, 39, pp. 371-378 (2000).
- ↑ A. W. C. Lau et al., "Microrheology, Stress Fluctuations, and Active Behavior of Living Cells," Phys. Rev. Lett., 91(19), p. 198101 (2003)
- ↑ Tseng et al. :Yiider Tseng, Thomas P Kole, and Denis Wirtz, "Micromechanical mapping of live cells by multiple-particle-tracking microrheology." Biophys J. 2002 December; 83(6): 3162–3176.