Difference between revisions of "Confocal Wiki"
(→Design) |
(→Design) |
||
| Line 1: | Line 1: | ||
| − | =Design= | + | =Measuring Morphology of Biological Specimen using a Low-Cost Confocal Microscope: An Undergraduate Teaching Experiment= |
| − | ==Optical Setup== | + | |
| + | Stephanie Bachar, Sivakami Sambasivam, Steven Wassermann and Steven Nagle. | ||
| + | |||
| + | ---- | ||
| + | |||
| + | =1 Motivation= | ||
| + | ==1.1 Background and Motivation== | ||
| + | The main advantage of a confocal microscope is its ability to image a very narrow depth of field, advantageously excluding light from above or below the focal plane of the image. This is done by using a pinhole, strategically placed where the light from the focal plane comes to a focal point while light from below and above the sample is converging or diverging. As a result, confocal microscopes have increased contrast compared to wide-field microscopes. Additionally, they can be used to generate 3D images by taking a series of 2-dimensional images along the z-axis of a specimen. Also, confocal microscopes have a marginally higher resolution than wide-field microscopes. | ||
| + | |||
| + | Currently, confocal microscopes cost on the order of $100,000 - quite an expensive purchase for a teaching laboratory or a low budget laboratory. Recently, there has been a new advent of creating low-cost imaging systems to allow for higher quality imaging in resource constrained environments. Xi et. al (2006) created a $8561 addition to a $20,000 inverted microscope allowing confocal imaging at a much lower cost than confocal microscopes available on the market. Further, researchers at the Massachusetts Institute of Technology have created an atomic force microscope that costs less than 10% of research-grade atomic force microscopes and other MIT researchers have created a cost-efficient optical trap for use by undergraduates. | ||
| + | |||
| + | The objective of this project is to create a teaching confocal microscope with associated documentation and suggested experiments for approximately $20,000. Based on our literature review, such a system does not currently exist. | ||
| + | |||
| + | ==1.2 Previous MIT Prototypes== | ||
| + | In Fall 2009 of 20.309, Laskey, Ramanthan, Rurak and Wilder began the construction of a confocal microscope in the 20.309 laboratory. Stephanie Bachar continued work on the microscope during January of 2011. When beginning our project, the confocal microscope was functional but had a number of imaging and processing limitations. There were also a number of additional components which could be added and characterizations which could be performed to augment the device. | ||
| + | |||
| + | There were some stability issues in the optical setup, as well as unnecessarily complex construction. The signal generated by a fluorescent reference slide was about 2V, which is somewhat low. The images generated by the confocal microscope had blurry edges and inconsistent intensity due to fluctuations in the laser output. While there had been successfully imaged patterns on a fluorescent reference slide, the setup was unable to pick up signal from fluorescent beads. This was likely due to the resolution capabilities of the microscope as well as the low density and dimmed fluorescence of the beads used. | ||
| + | Matlab code existed to generate a laser scan over the sample and to recompose the associated output from the PMT into an image. However, the scanning and re-composition code was quite inefficient, resulting in very slow image generation. Furthermore, the scan code generated a single 2D image of the sample instead of 3D imaging. | ||
| + | |||
| + | ---- | ||
| + | |||
| + | =2 Design= | ||
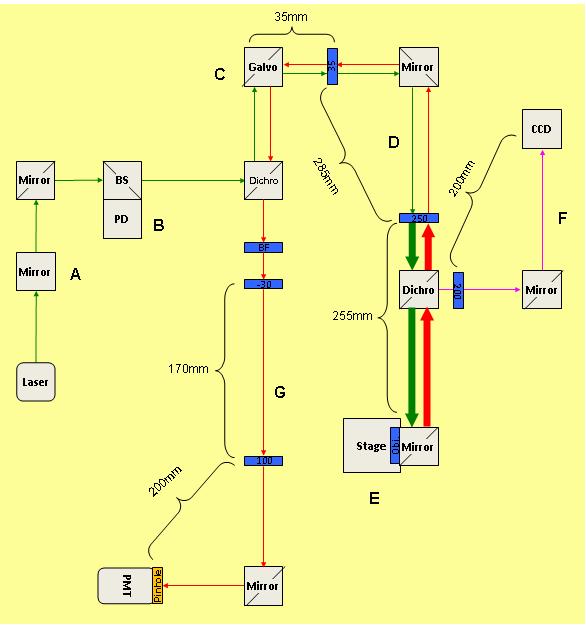
| + | ==2.1 Optical Setup== | ||
[[File:Beam path confocal.JPG|center|thumb|600px]] | [[File:Beam path confocal.JPG|center|thumb|600px]] | ||
Revision as of 22:24, 23 May 2011
Contents
Measuring Morphology of Biological Specimen using a Low-Cost Confocal Microscope: An Undergraduate Teaching Experiment
Stephanie Bachar, Sivakami Sambasivam, Steven Wassermann and Steven Nagle.
1 Motivation
1.1 Background and Motivation
The main advantage of a confocal microscope is its ability to image a very narrow depth of field, advantageously excluding light from above or below the focal plane of the image. This is done by using a pinhole, strategically placed where the light from the focal plane comes to a focal point while light from below and above the sample is converging or diverging. As a result, confocal microscopes have increased contrast compared to wide-field microscopes. Additionally, they can be used to generate 3D images by taking a series of 2-dimensional images along the z-axis of a specimen. Also, confocal microscopes have a marginally higher resolution than wide-field microscopes.
Currently, confocal microscopes cost on the order of $100,000 - quite an expensive purchase for a teaching laboratory or a low budget laboratory. Recently, there has been a new advent of creating low-cost imaging systems to allow for higher quality imaging in resource constrained environments. Xi et. al (2006) created a $8561 addition to a $20,000 inverted microscope allowing confocal imaging at a much lower cost than confocal microscopes available on the market. Further, researchers at the Massachusetts Institute of Technology have created an atomic force microscope that costs less than 10% of research-grade atomic force microscopes and other MIT researchers have created a cost-efficient optical trap for use by undergraduates.
The objective of this project is to create a teaching confocal microscope with associated documentation and suggested experiments for approximately $20,000. Based on our literature review, such a system does not currently exist.
1.2 Previous MIT Prototypes
In Fall 2009 of 20.309, Laskey, Ramanthan, Rurak and Wilder began the construction of a confocal microscope in the 20.309 laboratory. Stephanie Bachar continued work on the microscope during January of 2011. When beginning our project, the confocal microscope was functional but had a number of imaging and processing limitations. There were also a number of additional components which could be added and characterizations which could be performed to augment the device.
There were some stability issues in the optical setup, as well as unnecessarily complex construction. The signal generated by a fluorescent reference slide was about 2V, which is somewhat low. The images generated by the confocal microscope had blurry edges and inconsistent intensity due to fluctuations in the laser output. While there had been successfully imaged patterns on a fluorescent reference slide, the setup was unable to pick up signal from fluorescent beads. This was likely due to the resolution capabilities of the microscope as well as the low density and dimmed fluorescence of the beads used. Matlab code existed to generate a laser scan over the sample and to recompose the associated output from the PMT into an image. However, the scanning and re-composition code was quite inefficient, resulting in very slow image generation. Furthermore, the scan code generated a single 2D image of the sample instead of 3D imaging.
2 Design
2.1 Optical Setup
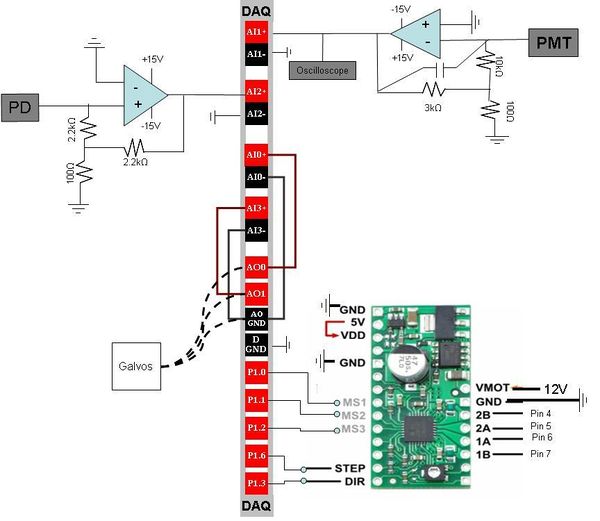
Circuit Design
Stepper Motor Driver The stepper motor driver is powered by a 12V power supply. The driver takes output voltage from the DAQ and converts it into signals that moves the stepper motor that controls the stage in the Z direction. 2B and 2A connect to coil 2 and 1A and 1B connect to coil 1 of the stepper motor.
INSERT a chart below!!
- Presumably the Limit Switch pins allow you to set a max/min voltage to the stepper motor to prevent damaging the motor. We have not yet figured out how this works. However, the pins are connected to the DAQ so that the output can be read and understood.
When STEP turns high/low, the motor takes a step. DIR controls the direction (up or down) of the stepper motor. High = up, Low = down. MS1, MS2, MS3 allow for fractional steps, up to 1/16th of a full step.
The maximum current tolerated by the stepper motor is 1A. The current output by the stepper motor driver is given by the equation:
Where RS = 0.05 Ω and VREF is the output of the 6th pin from the top left of the stepper motor driver (one below GND, two above MS1). The output the VREF can be adjusted by screwing Δ. ITripMAX should be less than 1A. We worked with ITripMAX as ~0.5A, or VREF ~ 0.2V.
FIGURE OUT HOW TO INSERT EQUATIONSItalic text (otherwise bring them in as pictures) Photodiode Correction When visualizing the laser signal, it was apparent from analyzing the noise that the variation of the laser signal was stochastic. To correct for the propagation of this noise to the value of fluorescence we measured through the PMT, we utilized a photodiode. We placed a beam splitter that would divert a portion of the light into the photodiode. We then used the photodiode output to attempt to correct the PMT output.
The PMT output is characterized as
〖PMT〗_outputi=m_3 (I_(∆_i )+ I_(b_i ) )*N_(f_i )+ PMT(I=∅)
with m_3 representing the conversion factor from intensity to voltage for the PMT, N_(f_i ) representing the number of fluorophores in the sample, I_(∆_i ) representing the change in intensity due to the stochastic variation of the laser and I_(b_i ) representing the intensity of the baseline laser signal. PMT(I=∅) represents the offset of the PMT value.
The PD output is characterized as
〖PD〗_outputi=m_1 (I_(∆_i )+ I_(b_i ) )+ PD(I=∅) with m_1 representing the conversion factor from intensity to voltage for the PD, I_(∆_i ) representing the change in intensity due to the stochastic variation of the laser and I_(b_i ) representing the intensity of the baseline laser signal and the fluorescence in the sample. PD(I=∅) represents the offset of the PMT value. A rearrangement of these equations results in the final formula
〖PMT〗_outputi-PMT(I=∅)=m_3/m_1 (〖PD〗_outputi-PD(I=∅))*N_(f_i ) Our goal is to use our PMToutput and our PD output to find the value of N_(f_i )which will provide us with the value of the intensity of fluorophores in each location in our image. If we subtract the PMT offset from the PMT reading and proceed similarly with subtracting the PD offset from the PD reading, we can calculate ΔPMT and ΔPD.
∆PMT/∆PD= m_3/m_1 *N_(f_i ) Since we will be rescaling the N_(f_i )so that the range of values is 0 to 1, we do not need to worry about the m3 and m1 factors assuming that they will vary constantly with different intensities, an assumption that future students should confirm. If this assumption proves to be incorrect, the scaling factors of m3 and m1 should be calculated separately.
Original Image
Corrected Image using the Photodiode