Difference between revisions of "Assignment 8, Part 2: fabricate a microfluidic device"
MAXINE JONAS (Talk | contribs) (→Overview) |
MAXINE JONAS (Talk | contribs) (→Connect tubing) |
||
| Line 56: | Line 56: | ||
# Finally, in order to interface the Tygon tubing with the holes in your PDMS device, cut 3 x 1 cm sections of the thin orange PEEK tubing. | # Finally, in order to interface the Tygon tubing with the holes in your PDMS device, cut 3 x 1 cm sections of the thin orange PEEK tubing. | ||
# Sleeve a section of PEEK tubing into the short end of each inlet channel, and into the outlet channel. | # Sleeve a section of PEEK tubing into the short end of each inlet channel, and into the outlet channel. | ||
| − | # Connect the tubing assembly to the appropriate inlet and outlet | + | # Connect the tubing assembly to the appropriate inlet and outlet holes in the PDMS. |
==References== | ==References== | ||
Revision as of 13:46, 2 November 2018
Overview
In this part of the assignment, you will make a microfluidic device out of double-sided tape sandwiched between a glass coverslip and a slab of PDMS. This procedure is incredibly simple, as far as microfluidics go, and was developed in Paul Blainey's lab to image the motion of transport molecules along DNA [1].
PDMS is a silicone elastomer made by mixing together a viscous liquid base with a crossliking agent. Once mixed and annealed at 60°C, the material will harden into a solid, rubber-like material that is optically clear, non-toxic, and chemically inert. Researchers typically use PDMS for microfluidics because they can use it to cast very small sharp features (down to ~1 micron), and they can covalently bond the PDMS to a glass coverslip, creating a sealed device that is readily compatible with most types of microscopy.
Since our experiment does not require extremely small features, we will simply cut the Y-shaped channel out of a piece of double-sided tape using a vinyl cutter, rather than casting PDMS over a mold. We're still going to take advantage of PDMS's properties, though. First, it will provide an optically clear structure for our device, and second, because it is somewhat soft and flexible, we can easily punch holes in it (which is hard to do with something like glass) in order to connect our flow channel to inlet and outlet tubing.
Cast a slab of PDMS
Two notes before you begin:
- This protocol has two steps with 30-minute wait times. Part 2 of this assignment can be completed in parallel with Part 1, if you want to keep making progress during the downtime.
- PDMS is not harmful, but it is viscous and sticky. Wear gloves when pouring the elastomer base and crosslinking agent, and change them before touching other lab equipment. If you spill any, clean it up right away with a paper towel or kimwipe.
Onward!
- Turn on the oven to 60°C (if it isn't on already).
- Using the scale, measure 31.5 g of the Sylgard™ 184 Silicone Elastomer base into a paper cup.
- Pour in 3.5 g of the curing agent to total 35 g. Note that the curing agent is much less viscous than the base, so pour extra carefully so you don't add too much.
- Mix the elastomer base and curing agent REALLY WELL (i.e. > 1 minute) using a plastic stirrer.
- Pour the mixture into a square petri dish.
- Degas the PDMS for 30 minutes using the vacuum desiccator. Make sure the red T-valve is closed before turning on the vacuum.
- After 30 minutes, turn off the vacuum to the desiccator, and slowly vent the chamber using the red T-valve.
- Remove ALL remaining bubbles with a plastic stirrer.
- Place the petri dish in the 60°C oven for 30 minutes.
The procedure can be stopped at this point and you may store the cured PDMS in the petri dish if you do not have time to complete the next steps in the procedure right away.
Assemble your device
- Work on a cutting mat, and not the bench, when punching holes and cutting tubing.
- Remove a rectangle of pre-cut double-sided tape from the large sheet. (Do not yet remove the clear backing of the tape!)
- Cut out a slab of PDMS roughly the same size as the tape, or slightly larger. Leave it in the dish until you are ready to use it, to prevent dust accumulation.
- You should be able to cut at least 6 devices out of a single petri dish. You will need to make more of the same devices in future assignments, so plan accordingly.
- Using tweezers, remove the internal Y-shape from the cut tape. Check that you've removed the clear backing of the Y-shape as well as the green tape.
- Use tweezers to peel off the clear backing from ONE side of the tape. Remove the cut PDMS slab from the petri dish, and stick the flat side (bottom) onto the green tape. Press everywhere to ensure a full seal.
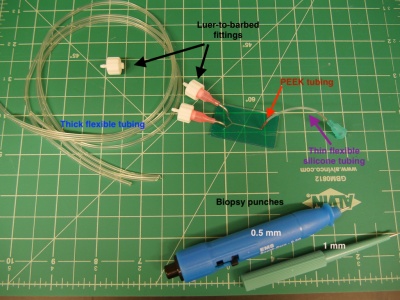
- Use a 0.5 mm biopsy punch to make holes for the inlet and outlet tubing. After inserting the biopsy punch, remove the small core that it creates before extracting the punch from the PDMS.
- Peel off the clear backing from the other side of the tape and seal the flow channel to a 22x40 mm glass coverslip. Apply pressure to the PDMS, rather than the coverslip, to prevent cracking of the coverslip.
Your device is now assembled!
Connect tubing
- Using a scalpel, cut the following lengths of Tygon tubing (OD 0.06", ID 0.02"). Cutting the tubing on an angle will make it easier to sleeve together.
- 2 x 5" lengths and
- 2 x 10" lengths for the inlets, and
- one 15" length for the outlet.
- Additionally, cut 2 x 2.5" lengths of the flexible silicone tubing for the inlets.
- Sleeve together the two inlet tubings, each consisting of: one 10" length of Tygon, one 2.5" length of silicone, and one 5" length of Tygon tubing.
- Finally, in order to interface the Tygon tubing with the holes in your PDMS device, cut 3 x 1 cm sections of the thin orange PEEK tubing.
- Sleeve a section of PEEK tubing into the short end of each inlet channel, and into the outlet channel.
- Connect the tubing assembly to the appropriate inlet and outlet holes in the PDMS.
References
- ↑ | K. Xiong and P. C. Blainey, “A Simple, Robust, and High Throughput Single Molecule Flow Stretching Assay Implementation for Studying Transport of Molecules Along DNA,” J. Vis. Exp., no. 128, pp. 1–7, 2017
- Overview
- Part 1: feedback systems
- Part 2: fabricate a microfluidic device
- Part 3: add flow control and test your device
Back to 20.309 Main Page