20.109(S22):M2D2
Introduction
In this module you will engineer the metabolism of E. coli in an attempt to increase the production of a commercially relevant product. Specifically, use will use metabolic engineering to increase either the ethanol yield or acetate yield via manipulating the fermentation pathway native to E. coli cells using CRIPSRi.
Ethanol, or ethyl alcohol, is used as fuel – most commonly as a biofuel additive in gasoline. Current methods for producing ethanol involve the use of agricultural feedstocks and concern is mounting given the effect this may have on food prices and resources. Using E. coli as an ethanol-generating machine is promising; however, the ethanol output of a native cell is very low.
Acetate is a salt combined that results from the combination of acetic acid with an alkaline, earthy, or metallic base. The majority of the 5 billion kilograms of acetic acid produced each year by industry are used to produce acetates, which are then used in the production of polymers. As with ethanol, the use of E. coli to generate lactate is of interest, but the amount produced by a native cell is low.
Metabolic engineering refers to the alteration of genetic and/or regulatory circuitry within organisms. The native circuitry involves a series of enzymes that perform biochemical reactions that function to convert raw substrates into products that are required for the organism’s survival. When this native circuitry is altered using metabolic engineering techniques, the goal is to use the host organisms as machines that produce valuable materials in large quantities and at low cost.
Common strategies employed in metabolic engineering are:- Increasing expression of a gene that encodes an enzyme responsible for a rate-limiting step.
- Inhibiting competing pathways that divert substrate away from the pathway of interest.
- Incorporating genes from other organisms into the host.
- Altering the structure of the enzyme such that yield is increased.
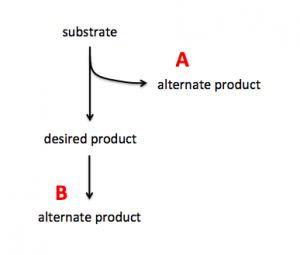
To increase production of a desired product, you can either target proteins that use the substrate to generate alternate products (see A in image on right) or you can target proteins that use the desired product to generate alternate products (see B in image on right). In A the substrate is siphoned away from the reaction that generates the desired product and in B the desired product is used as substrate in a subsequent reaction. By eliminating the proteins that catalyze the reactions that result in alternate products, you can potentially increase production of your desired product.
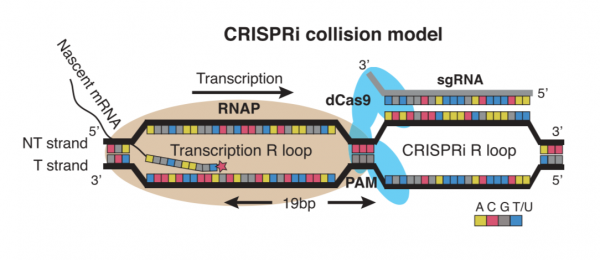
To engineer the metabolism of E. coli, you will use the CRISPRi system. Unlike more commonly used CRISPR-based technologies, CRISPRi is used to modulate expression from the genome rather than to modify the genome. This distinction is due to the use of an enzymatically-inactive dCas9 (or ‘dead’ Cas9) protein. Because dCas9 is enzymatically inactive, it is unable to cleave the DNA upon binding to the targeted sequence in the host genome. The lack of DNA cleavage results in gene silencing through impeding RNA polymerase binding, transcription factor binding, and/or transcription elongation. This method of repression referred to as CRISPRi collision and depicted in the schematic below.