Difference between revisions of "20.109(S16):Site-directed mutagenesis (Day3)"
MAXINE JONAS (Talk | contribs) (Created page with "{{Template:20.109(S16)}} <div style="padding: 10px; width: 790px; border: 5px solid #33CC66;"> ==Introduction== The process of scientific inquiry encompasses much more than...") |
Noreen Lyell (Talk | contribs) (→Part 1: BE Communication Lab workshop) |
||
| (28 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
==Introduction== | ==Introduction== | ||
| − | + | [[Image:20.109_SDM-Nobel.png|thumb|right|200px| 1993 Chemistry Nobel Prize co-winner (with Kary Mullis, inventor of PCR) for developing site-directed mutagenesis.]] | |
| − | + | Last time you used both two- and three-dimensional protein information to design primers that incorporate a mutation into the wild-type inverse pericam protein. Today you will use site-directed mutagenesis (SDM) to incorporate the corresponding base pair changes. The SDM strategy you will use shares some features with the polymerase chain reaction (PCR) for DNA amplification. Recall from Day 1 that PCR amplification involves multiple cycles of melting, annealing, and extending. To incorporate base-pair mutations in the product DNA, primers that have a slight mismatch to the original template can be used. At a low enough annealing temperature (~25 °C below the primer melting temperature as defined for mutagenesis), these nearly-complementary primers will still anneal to the template DNA, but the copies created during the extension phase will contain the mutation. | |
| − | + | Before we continue our discussion of SDM, we should think more about how the sequences you designed were used to generate actual primers that can be used to amplify and, in SDM, incorporate mutations in DNA. Current oligonucleotide (primer) synthesis uses phosphoramidite monomers, which are simply nucleotides with protection groups added. The protection groups prevent side reactions and promote the formation of the correct DNA product. The DNA product synthesis starts with the 3' -most nucleotide and cycles through four steps: deprotection, coupling, capping, and stabilization. First, deprotection removes the protection groups. Second, during coupling the 5' to 3' linkage is generated with the incoming nucleotide. Next, a capping reaction is completed to prevent uncoupled nucleotides from forming unwanted byproducts. Lastly, stabilization is achieved through an oxidation reaction that makes the phosphate group pentavalent. For a detailed description of this process, read [[Media:IDT chemical-synthesis-of-oligonucleotides.pdf |this article]] from IDT DNA. | |
| − | + | You will combine the mutagenic primers you designed with plasmid DNA encoding wild-type inverse pericam (pRSET-IPC). DNA polymerase will copy the plasmid using the mutagenic primers to generate a product that carries the X#Z mutated inverse pericam gene. Following this reaction the mutated product is a linear DNA fragment. To generate circular plasmids that carry the X#Z IPC gene, the DNA is phosphorylated then ligated. In addition, there is still parental -- that is, non-mutant -- DNA present in your SDM reaction product. To ensure that ''only'' the mutant plasmid is used in the next steps, the parental DNA is selectively digested using the ''DpnI'' enzyme. The underlying selective property is that ''DpnI'' only digests methylated DNA. Therefore, the synthetically made (and thus non-methylated) mutant DNA is not digested, while the parental DNA is digested due to methylation by the host bacterial strain originally used to amplify it. The resulting small linear pieces of parental DNA are simply degraded by the bacteria upon transformation, whereas the intact (due to the phosphorylation and ligation reaction) circular mutant DNA is amplified by the bacteria. | |
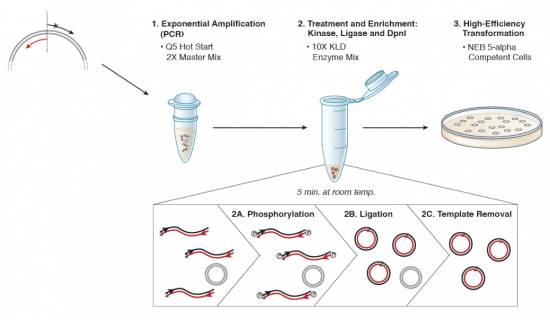
| − | + | [[Image:Sp16 M1D3 SDM schematic.png|thumb|center|550px|'''Schematic of NEB Q5 Site Directed Mutagenesis Kit procedure modified from NEB manual.''']] | |
| − | + | ||
| − | [[Image: | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<br style="clear:both;"/> | <br style="clear:both;"/> | ||
| − | + | After today's lab session, the teaching faculty will transform your mutated plasmids into cells that are able to generate multiple copies. When you return you will receive a liquid culture of bacterial cells that contain your, hopefully mutated, plasmid. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | After today's lab session, the teaching faculty will transform your mutated plasmids into cells that are able to generate multiple copies. When you return you will receive your | + | |
==Protocols== | ==Protocols== | ||
| − | ===Part 1: | + | ===Part 1: BE Communication Lab workshop=== |
| − | + | Throughout the semester you will present the data you generate as both written and oral communications. Figures and the corresponding captions are tools used to convey your data to an audience, and will be very important components of your Mod 1 Protein engineering summary and Mod 2 Systems research article. To prepare you for this task, today we will be joined by Dr. Diana Chien and Dr. Vivian Siegel to discuss how to design effective Figures and Captions. | |
| − | [[ | + | Please find the [[Media:20.109 Workshop 1 - Figures 2016.pdf| Slide Presentation]] and a [[Media:Figure, Caption Rubric.pdf| Rubric]] from the BE Communication Lab to assist you with your assignments. |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ===Part 2: Primer preparation=== | |
| + | While you were away the sequences for the mutagenic primers you designed were submitted to Integrated DNA Technologies (IDT). IDT synthesized the primers then lyophilized (dried) them to a powder. Follow the steps below to resuspend your primers. | ||
| + | #Centrifuge the tubes containing your lyophilized primers for 1 min. | ||
| + | #Calculate the amount of water needed ''for each primer'' (forward and reverse, separately) to give a concentration of 100 μM. | ||
| + | #Resuspend each primer stock in the appropriate volume of sterile water, vortex, and centrifuge. | ||
| + | #Now prepare a dilution from your archival stock. Prepare 100 μL of a solution that has both the forward and reverse primers, ''each'' primer at 10 μM. | ||
| + | #*Try the calculation on your own first. If you get stuck, ask the teaching faculty for help. | ||
| + | #*Be sure to change tips between primers! | ||
| + | #Return the rest of your primer stocks, plus your primer specification sheets, to the front bench. | ||
| − | ===Part | + | ===Part 3: Site-directed mutagenesis=== |
| − | + | We will be using the Q5 Site Directed Mutagenesis Kit from NEB to perform your site-directed mutagenesis reactions. Each group will set up one reaction, for your X#Z mutation. Meanwhile, the teaching faculty will set up a single positive control reaction, to ensure that all the reagents are working properly. You should work quickly but carefully, and keep your tube in a chilled container at all times. '''Please return shared reagents to the ice bucket(s) from which you took them as soon as you are done with each one.''' | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | We will be using the Q5 Site Directed Mutagenesis Kit from NEB to perform your site-directed mutagenesis reactions. Each group will set up one reaction, for | + | |
#Get a PCR tube and label the top with your mutation and lab section (write small!). | #Get a PCR tube and label the top with your mutation and lab section (write small!). | ||
#Add 10.25 μL of nuclease-free water. | #Add 10.25 μL of nuclease-free water. | ||
| − | #Add 1.25 μL of your mutagenesis primer mix ( | + | #Add 1.25 μL of your mutagenesis primer mix (each primer should be at a concentration of 10 μM). |
| − | #Add 1 μL of IPC template DNA ( | + | #Add 1 μL of IPC template DNA (concentration of 25 ng/μL). |
#Lastly, use a filter tip to add 12.5 μL of Q5 Hot Start High-Fidelity 2X Master Mix - containing buffer, dNTPs, and polymerase - to your tube. | #Lastly, use a filter tip to add 12.5 μL of Q5 Hot Start High-Fidelity 2X Master Mix - containing buffer, dNTPs, and polymerase - to your tube. | ||
| − | #Once | + | #Once all groups are ready, we will begin the thermocycler, under the following conditions: |
<center> | <center> | ||
| Line 241: | Line 92: | ||
#Add 5 μL of KLD mix to 50 μL of chemically-competent NEB 5α. | #Add 5 μL of KLD mix to 50 μL of chemically-competent NEB 5α. | ||
#Incubate on ice for 30 min. | #Incubate on ice for 30 min. | ||
| − | #Heat shock at 42 °C for 30 | + | #Heat shock at 42 °C for 30 s. |
#Incubate on ice for 5 min. | #Incubate on ice for 5 min. | ||
| − | #Add 950 μL SOC and gently shake at 37 °C for 1 | + | #Add 950 μL SOC and gently shake at 37 °C for 1 h. |
| − | #Spread 50 μL onto LB | + | #Spread 50 μL onto LB+Amp plate and incubate overnight at 37 °C. |
| + | |||
| + | ===Part 4: Journal article discussion=== | ||
| + | |||
| + | The purpose of this discussion will be two-fold: 1) to familiarize ourselves with the history of protein design, and 2) to continue to explore ways of talking about the scientific literature. | ||
| + | |||
| + | If you chose to read the paper by [http://www.ncbi.nlm.nih.gov/pubmed/7809066?ordinalpos=9&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum Heim, Prasher, and Tsien], consider the following questions. | ||
| + | |||
| + | *What are the advantages of GFP compared to synthetic fluorescent dyes, and what are its limitations? Which of these limitations are Heim et al. trying to address?<br> | ||
| + | *What methods did the authors use for mutagenesis, and how do they compare to the method we are using? <br> | ||
| + | *How were mutagenic proteins initially selected, and how were the chosen ones further analyzed? <br> | ||
| + | *What is the significance of the different wild-type protein fractions shown in Figure 1? <br> | ||
| + | *If GFP maturation does not require any cofactors for a chemical reaction, why does it take four hours? What lines of evidence suggest the absence of cofactors? <br> | ||
| + | *In what part(s) of the protein were useful amino acid substitutions found? <br> | ||
| + | |||
| + | We will not have the opportunity to discuss these questions as a class, but feel free to ask the teaching faculty any questions you may have concerning the journal article. | ||
| + | |||
| + | You should be familiar with the whole [http://www.ncbi.nlm.nih.gov/pubmed/11248055?ordinalpos=5&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum Nagai et al] paper, but your team will only be asked to explain/describe the portion assigned below. Use the questions associated with your section to guide you as you prepare for the group discussion. | ||
| + | |||
| + | #'''GROUP DISCUSSION''' Introduction, Methods (gene construction) and Figure 1 | ||
| + | #*Consider: What is a chimeric protein? A circularly permuted one? | ||
| + | #*How does the chimeric protein pericam work as a sensor? (No need for details about different types of pericams yet.) | ||
| + | #*Briefly summarize how pericam was constructed at the gene level. What changes had to be made to express pericam in mammalian rather than bacterial cells? | ||
| + | #<font color = #FF0000>'''T/R RED TEAM'''</font color> and '''W/F GROUP DISCUSSION''' Figure 2 | ||
| + | #*What do the authors learn about cpEYFP structure from the absorbance spectra? | ||
| + | #*Compare the wavelength used for testing pericam with the excitation maximum of cpEYFP. Why might they be slightly different? | ||
| + | #*Besides making the critical mutations shown in Table 1, what did the authors have to do to make a working (fluorescent, calcium-sensitive) pericam? | ||
| + | #*What was the dynamic range (intensity change with calcium addition) of the first working cpEYFP? | ||
| + | #<font color = #FF6600>'''T/R ORANGE TEAM'''</font color> and <font color = #FF0000>'''W/F RED TEAM'''</font color> Table 1 (mutations) and Figure 3, A-I (focus on D-F) | ||
| + | #*Describe the three types of pericam initially constructed and tested and how they respond to calcium. | ||
| + | #*What kinds of amino acid substitutions were made, and why might they cause the noted effects? | ||
| + | #<font color = #FFFF33>'''T/R YELLOW TEAM'''</font color> and <font color = #FF6600>'''W/F ORANGE TEAM'''</font color> Table 1 (''K''<sub>d</sub>) and Figure 3, J-L | ||
| + | #*This set of figures is very similar to the one you will eventually create for your lab reports. It is not described at length in the text, so take a moment to decipher the axes and results as best you can, using outside resources if necessary. | ||
| + | #<font color = #00FF00>'''T/R GREEN TEAM'''</font color> and '''W/F GROUP DISCUSSION''' Figure 4 | ||
| + | #*How does flash-pericam improve upon previous limitations to imaging calcium in the nucleus and cytosol? | ||
| + | #*What chemicals can be used to create step-changes in intracellular calcium, and how might they work? | ||
| + | #<font color = #3333FF>'''BLUE TEAM'''</font color> Figure 5 | ||
| + | #*How did the authors calibrate calcium levels? | ||
| + | #*Compared to the other pericams, what's special about the way split-pericam functions? | ||
| + | #*What are the limitations of using split-pericam as a calcium sensor? | ||
| + | #<font color = #FF66CC>'''PINK TEAM'''</font color> Figure 6 | ||
| + | #*What modifications were made to pericam for organelle-level study, and how does the use of pericams improve upon previous procedures? | ||
| + | #*What were the authors able to learn about calcium transients in different organelles? | ||
| + | #<font color = #9900CC>'''PURPLE TEAM'''</font color> Wrap-up | ||
| + | #*Describe some other (not pericam-based) calcium indicators. | ||
| + | #*How does FRET work, and what are the pros/cons of using FRET-based sensors? | ||
| + | |||
| + | Finally, you should all consider the similarities and differences between the research described in the paper above and the research that you are undertaking in this module. | ||
| − | =Reagent list= | + | ==Reagent list== |
*Q5 Site Directed Mutagenesis Kit from NEB | *Q5 Site Directed Mutagenesis Kit from NEB | ||
| Line 255: | Line 153: | ||
***Proprietary mix of kinase, ligase, and ''DpnI'' enzymes. | ***Proprietary mix of kinase, ligase, and ''DpnI'' enzymes. | ||
*The SOC medium contains 2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, and 20 mM glucose. | *The SOC medium contains 2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, and 20 mM glucose. | ||
| − | * | + | *LB+Amp plates |
| + | **The Luria-Bertani (LB) broth contains 1% tryptone, 0.5% yeast extract, and 1% NaCl | ||
| + | **Plates prepared by adding 1.5% agar and 100 μg/mL ampicillin to LB | ||
| − | =Navigation links= | + | ==Navigation links== |
Next day: [[20.109(S16):Prepare expression system (Day4)| Prepare expression system]] <br> | Next day: [[20.109(S16):Prepare expression system (Day4)| Prepare expression system]] <br> | ||
Previous day: [[20.109(S16):Design mutation primers (Day2)| Design mutation primers]] | Previous day: [[20.109(S16):Design mutation primers (Day2)| Design mutation primers]] | ||
Latest revision as of 14:08, 17 February 2016
Contents
Introduction
Last time you used both two- and three-dimensional protein information to design primers that incorporate a mutation into the wild-type inverse pericam protein. Today you will use site-directed mutagenesis (SDM) to incorporate the corresponding base pair changes. The SDM strategy you will use shares some features with the polymerase chain reaction (PCR) for DNA amplification. Recall from Day 1 that PCR amplification involves multiple cycles of melting, annealing, and extending. To incorporate base-pair mutations in the product DNA, primers that have a slight mismatch to the original template can be used. At a low enough annealing temperature (~25 °C below the primer melting temperature as defined for mutagenesis), these nearly-complementary primers will still anneal to the template DNA, but the copies created during the extension phase will contain the mutation.
Before we continue our discussion of SDM, we should think more about how the sequences you designed were used to generate actual primers that can be used to amplify and, in SDM, incorporate mutations in DNA. Current oligonucleotide (primer) synthesis uses phosphoramidite monomers, which are simply nucleotides with protection groups added. The protection groups prevent side reactions and promote the formation of the correct DNA product. The DNA product synthesis starts with the 3' -most nucleotide and cycles through four steps: deprotection, coupling, capping, and stabilization. First, deprotection removes the protection groups. Second, during coupling the 5' to 3' linkage is generated with the incoming nucleotide. Next, a capping reaction is completed to prevent uncoupled nucleotides from forming unwanted byproducts. Lastly, stabilization is achieved through an oxidation reaction that makes the phosphate group pentavalent. For a detailed description of this process, read this article from IDT DNA.
You will combine the mutagenic primers you designed with plasmid DNA encoding wild-type inverse pericam (pRSET-IPC). DNA polymerase will copy the plasmid using the mutagenic primers to generate a product that carries the X#Z mutated inverse pericam gene. Following this reaction the mutated product is a linear DNA fragment. To generate circular plasmids that carry the X#Z IPC gene, the DNA is phosphorylated then ligated. In addition, there is still parental -- that is, non-mutant -- DNA present in your SDM reaction product. To ensure that only the mutant plasmid is used in the next steps, the parental DNA is selectively digested using the DpnI enzyme. The underlying selective property is that DpnI only digests methylated DNA. Therefore, the synthetically made (and thus non-methylated) mutant DNA is not digested, while the parental DNA is digested due to methylation by the host bacterial strain originally used to amplify it. The resulting small linear pieces of parental DNA are simply degraded by the bacteria upon transformation, whereas the intact (due to the phosphorylation and ligation reaction) circular mutant DNA is amplified by the bacteria.
After today's lab session, the teaching faculty will transform your mutated plasmids into cells that are able to generate multiple copies. When you return you will receive a liquid culture of bacterial cells that contain your, hopefully mutated, plasmid.
Protocols
Part 1: BE Communication Lab workshop
Throughout the semester you will present the data you generate as both written and oral communications. Figures and the corresponding captions are tools used to convey your data to an audience, and will be very important components of your Mod 1 Protein engineering summary and Mod 2 Systems research article. To prepare you for this task, today we will be joined by Dr. Diana Chien and Dr. Vivian Siegel to discuss how to design effective Figures and Captions.
Please find the Slide Presentation and a Rubric from the BE Communication Lab to assist you with your assignments.
Part 2: Primer preparation
While you were away the sequences for the mutagenic primers you designed were submitted to Integrated DNA Technologies (IDT). IDT synthesized the primers then lyophilized (dried) them to a powder. Follow the steps below to resuspend your primers.
- Centrifuge the tubes containing your lyophilized primers for 1 min.
- Calculate the amount of water needed for each primer (forward and reverse, separately) to give a concentration of 100 μM.
- Resuspend each primer stock in the appropriate volume of sterile water, vortex, and centrifuge.
- Now prepare a dilution from your archival stock. Prepare 100 μL of a solution that has both the forward and reverse primers, each primer at 10 μM.
- Try the calculation on your own first. If you get stuck, ask the teaching faculty for help.
- Be sure to change tips between primers!
- Return the rest of your primer stocks, plus your primer specification sheets, to the front bench.
Part 3: Site-directed mutagenesis
We will be using the Q5 Site Directed Mutagenesis Kit from NEB to perform your site-directed mutagenesis reactions. Each group will set up one reaction, for your X#Z mutation. Meanwhile, the teaching faculty will set up a single positive control reaction, to ensure that all the reagents are working properly. You should work quickly but carefully, and keep your tube in a chilled container at all times. Please return shared reagents to the ice bucket(s) from which you took them as soon as you are done with each one.
- Get a PCR tube and label the top with your mutation and lab section (write small!).
- Add 10.25 μL of nuclease-free water.
- Add 1.25 μL of your mutagenesis primer mix (each primer should be at a concentration of 10 μM).
- Add 1 μL of IPC template DNA (concentration of 25 ng/μL).
- Lastly, use a filter tip to add 12.5 μL of Q5 Hot Start High-Fidelity 2X Master Mix - containing buffer, dNTPs, and polymerase - to your tube.
- Once all groups are ready, we will begin the thermocycler, under the following conditions:
| Segment | Cycles | Temperature | Time |
|---|---|---|---|
| Initial denaturation | 1 | 98 °C | 30 s |
| Amplification | 25 | 98 °C | 10 s |
| 55 °C | 30 s | ||
| 72 °C | 2 min | ||
| Final extension | 1 | 72 °C | 2 min |
| Hold | 1 | 4 °C | indefinite |
- After the cycling is completed, the teaching faculty will complete the KLD reaction (which stands for "kinase, ligase, DnpI") using 1 μL of your amplification product, 5 μL 2X KLD Reaction Buffer, 1 μL KLD Enzyme Mix, and 3 μL nuclease-free water. The reactions will be incubated for 5 min at room temperature.
- The teaching faculty will then use 5 μL of the KLD reaction product to complete a transformation into an E. coli strain (NEB 5α cells of genotype fhuA2 Δ(argF-lacZ)U169 phoA glnV44 Φ80 Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17) that will amplify the plasmid such that you are able to confirm the appropriate mutation was incorporated. The transformation procedure will be as follows:
- Add 5 μL of KLD mix to 50 μL of chemically-competent NEB 5α.
- Incubate on ice for 30 min.
- Heat shock at 42 °C for 30 s.
- Incubate on ice for 5 min.
- Add 950 μL SOC and gently shake at 37 °C for 1 h.
- Spread 50 μL onto LB+Amp plate and incubate overnight at 37 °C.
Part 4: Journal article discussion
The purpose of this discussion will be two-fold: 1) to familiarize ourselves with the history of protein design, and 2) to continue to explore ways of talking about the scientific literature.
If you chose to read the paper by Heim, Prasher, and Tsien, consider the following questions.
- What are the advantages of GFP compared to synthetic fluorescent dyes, and what are its limitations? Which of these limitations are Heim et al. trying to address?
- What methods did the authors use for mutagenesis, and how do they compare to the method we are using?
- How were mutagenic proteins initially selected, and how were the chosen ones further analyzed?
- What is the significance of the different wild-type protein fractions shown in Figure 1?
- If GFP maturation does not require any cofactors for a chemical reaction, why does it take four hours? What lines of evidence suggest the absence of cofactors?
- In what part(s) of the protein were useful amino acid substitutions found?
We will not have the opportunity to discuss these questions as a class, but feel free to ask the teaching faculty any questions you may have concerning the journal article.
You should be familiar with the whole Nagai et al paper, but your team will only be asked to explain/describe the portion assigned below. Use the questions associated with your section to guide you as you prepare for the group discussion.
- GROUP DISCUSSION Introduction, Methods (gene construction) and Figure 1
- Consider: What is a chimeric protein? A circularly permuted one?
- How does the chimeric protein pericam work as a sensor? (No need for details about different types of pericams yet.)
- Briefly summarize how pericam was constructed at the gene level. What changes had to be made to express pericam in mammalian rather than bacterial cells?
- T/R RED TEAM and W/F GROUP DISCUSSION Figure 2
- What do the authors learn about cpEYFP structure from the absorbance spectra?
- Compare the wavelength used for testing pericam with the excitation maximum of cpEYFP. Why might they be slightly different?
- Besides making the critical mutations shown in Table 1, what did the authors have to do to make a working (fluorescent, calcium-sensitive) pericam?
- What was the dynamic range (intensity change with calcium addition) of the first working cpEYFP?
- T/R ORANGE TEAM and W/F RED TEAM Table 1 (mutations) and Figure 3, A-I (focus on D-F)
- Describe the three types of pericam initially constructed and tested and how they respond to calcium.
- What kinds of amino acid substitutions were made, and why might they cause the noted effects?
- T/R YELLOW TEAM and W/F ORANGE TEAM Table 1 (Kd) and Figure 3, J-L
- This set of figures is very similar to the one you will eventually create for your lab reports. It is not described at length in the text, so take a moment to decipher the axes and results as best you can, using outside resources if necessary.
- T/R GREEN TEAM and W/F GROUP DISCUSSION Figure 4
- How does flash-pericam improve upon previous limitations to imaging calcium in the nucleus and cytosol?
- What chemicals can be used to create step-changes in intracellular calcium, and how might they work?
- BLUE TEAM Figure 5
- How did the authors calibrate calcium levels?
- Compared to the other pericams, what's special about the way split-pericam functions?
- What are the limitations of using split-pericam as a calcium sensor?
- PINK TEAM Figure 6
- What modifications were made to pericam for organelle-level study, and how does the use of pericams improve upon previous procedures?
- What were the authors able to learn about calcium transients in different organelles?
- PURPLE TEAM Wrap-up
- Describe some other (not pericam-based) calcium indicators.
- How does FRET work, and what are the pros/cons of using FRET-based sensors?
Finally, you should all consider the similarities and differences between the research described in the paper above and the research that you are undertaking in this module.
Reagent list
- Q5 Site Directed Mutagenesis Kit from NEB
- Q5 Hot Start High-Fidelity 2X Master Mix
- Propriety mix of Q5 Hot Start High-Fidelity DNA Polymerase, buffer, dNTPs, and Mg2+.
- 2X KLD Reaction Buffer
- 10X KLD Enzyme Mix
- Proprietary mix of kinase, ligase, and DpnI enzymes.
- Q5 Hot Start High-Fidelity 2X Master Mix
- The SOC medium contains 2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, and 20 mM glucose.
- LB+Amp plates
- The Luria-Bertani (LB) broth contains 1% tryptone, 0.5% yeast extract, and 1% NaCl
- Plates prepared by adding 1.5% agar and 100 μg/mL ampicillin to LB
Next day: Prepare expression system