20.109(F09): Mod 2 Day 3 Tools for system engineering
Contents
- 1 Tools for system engineering
Tools for system engineering
Introduction
Biological systems are not static. Thus they can be engineered to account for changing environmental conditions, as we've seen through our examination of two component regulatory systems. In addition, they can be engineered to account for changes that occur as the cells replicate and divide over time. Indeed, evolution of biological systems away from an original specification can be viewed as a curse (it's not like computer scientists have to worry that their software programs change functions when they relaunch them!) or a blessing (evolution can find a solution we didn't ever dream of). Here we'll take the rosy view and try to harness genetic variability to improve the bacterial photography system. In particular we'll screen a library of changes in the Cph8 gene to find ones that either increase the kinasing activity of the sensor when the cells are growing in the dark OR increase the phosphatase activity of the sensor when the cells are growing in the light. The first class of mutants (we'll call them K+) should make the "dark" color of the photographs more dark by increasing the amount of phosphorylated OmpR that stimulates LacZ transcription. The second class of mutants (we'll call these P+) should make the "light" color of the photographs lighter by decreasing the cell's levels of phosphorylated OmpR, thereby decreasing LacZ transcription.The region of the Cph8 protein to focus on for this purpose has been defined through traditional scientific studies of EnvZ, for example the work from Tom Silhavy's lab( PMID: 9721293 and pdf here). We've also been guided by the expertise of MIT's Mike Laub, whose lab studies the specificity and rewiring of two component regulatory systems. From these sources, a span of 5 contiguous amino acids can be identified as relevant for shifting the balance of EnvZ to greater kinasing or greater phosphatasing activity. These five residues in EnvZ are Alanine at amino acid 239 ("A239") through Histidine at amino acid 243 ("H243"), where mutations in the flanking residues (A239 and H243) have been shown to enhance the phosphatase activity of EnvZ and mutations in the internal residues (G240 V241 S242) enhance the kinase activity of EnvZ. The amino acid changes that modify the enzymatic activities are indicated on the figure below. Two important notes about these mutations though: First, the balance of kinase to phosphatase activities have been affected by the changes, but the mutations do not shift the reactions to fully "on" or fully "off." Second, the fusion protein of Cph1 to EnvZ, called Cph8, changes the numbering of the residues, as shown in the figure below. It's hoped, however, that the local environment of the region is similar to the natural EnvZ protein. 
To complement the genetic approach for solving biological engineering puzzles, we'll also consider two other approaches in synthetic biology. The first is a Registry of Standard Biological Parts, essentially a community resource that has some ready-made and useful genetic elements that can be assembled into synthetic biological devices systems. The second approach is to model biological systems, in this case we'll recapitulate the genetic structure of the bacterial photography system using electronic components, making explicit some of the benefits and limitations of such an approach.
Protocols
Part 1: Library Screen
The details for how the libraries were constructed and what kinds of changes are reasonable to expect will be considered in the next lab session. For today, you will transform a pool of DNA with degeneracies in the positions that affect the kinasing or phosphatasing activity of EnvZ. The recipient bacterial strain is identical to the bacterial photography system except that it does not harbor a plasmid encoding the light-sensing fusion protein Cph8. It does encode the OmpR-regulated LacZ gene as well as the phycobillins from a plasmid.
To carry out the transformation you will try your hand at a different technique for getting DNA into cells, namely electroporation. This method involves exposing a small but dense volume of cells to a pulse of current. The pulse momentarily flips the lipid bilayer, opening small spaces for the DNA to pass into the cells. As you can imagine, the cells aren't fond of such treatment and the single most important step to help them recover is to quickly add media to the cells once they've been electroporated. The process is also very sensitive to salts in the DNA, and if you pipet too much DNA to the cells, the extra salt may cause an electrical "arc," (you'll know this has happened from the flash of light and the "pop" you'll hear) frying the cells dead. If this happens, please get another aliquot of cells from the faculty and try the electroporation again, with less DNA.- When you are ready to electroporate the library, retrieve an aliquot of cells from the teaching faculty, a sterile cuvette, and an aliquot of rich, pre-warmed "SOC" media.
- Put the cuvette on ice.
- Pipet 2 ul of the library DNA that is being held in an icebucket on the teacher's bench into your aliquot of cells. Be sure to decide and then note if you are screening the K+ or the P+ library.
- Let the cells and the DNA incubate on ice one minute.
- Transfer 50 ul of the cells to the chilled cuvette and recover it with the blue lid.
- Put on your safety goggles.
- Tap the cuvette on the bench so the cells rest in the bottom of the cuvette.
- With the cuvette's "nub" facing forward, slide the cuvette into the electroporation chamber.
- Make sure the electroporator is set to "Ec2"
- Hold the pulse button until you hear a beep.
- Quickly remove the cuvette from the holder and quickly add the 0.5 ml volume of "SOC" media to the cells...
Part 2: Registry of Standard Biological Parts
Part 3: Modeling biological versus electrical devices
In this section, we'll explore the issue of signal strength in the context of an electrical circuit. As you work through this exercise, consider how the lessons learned from experimenting with an electronic circuit would map to the engineering of biological systems.
Acknowledgements
Many thanks to Tom Knight, Reshma Shetty and Barry Canton for this exercise, as well as Steve Wasserman for his troubleshooting and helpful improvements.
Safety information
In this exercise, you'll be working with circuits connected to power supplies. Practice good habits by never touching the circuit without first turning the power supply off (and verifying that it is off!).
System description
As you know, the bacterial photography system is constructed from two devices.
- An Input Sensing Device: a device which, in response to dark, produces a signal that's detected by the output generating device.
- An Output Generating Device: an actuator which takes an input signal from the first device and produces a detectable output.
We can construct an analogous system from electrical components.
- A photodiode: a sensor device that allows current to pass through it in response to incoming light
- An LED: an actuator that responds to a current input by emitting colored light.
We can thus use these two devices to build a circuit that will operate similarly to our bacterial photography system: the photodiode will sense light and generate an electrical signal that will be detected by the LED which produces a color.
System design
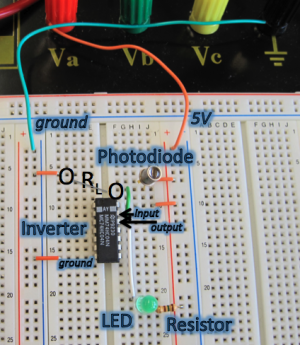
This system is fairly simple one since it only consists of a few components. In contrast to the bacterial photography system in which the signal is propagated through protein activities, here signals are propagated as either voltage or current. As you can see in the schematic, it contains the following parts.
- Photodiode: #MRD500: light sensor whose resistance decreases in the presence of light such that more current flows through it.
- LED: device which responds to a voltage drop across its terminals by emitting green-colored light.
- Resistor: components which resists current flow by producing a voltage drop across it.
- Inverter: #MM74HC04: a logic device which inverts its input signal.
The purpose of the photodiode is to serve as a light sensor (like Cph8 and the phycobilins) and the LED serves as a readout (like LacZ). The 150Ω resistor is there to ensure that the voltage drop across the LED isn't too high (which can cause the LED to breakdown). Notice the absence of a direct analog to this part in the bacterial photography system. The inverter provides a convenient way of connecting the photodiode to the LED and mimics the logic in the biological signaling cascade. The other resistor RL is there to ensure that the signal between the photodiode and inverter are matched, in much the same way as the opposing kinase/phosphatase activities temper the signal strength in the biological system.
When light shines on the photodiode, its resistance is decreased and current flows through it. Thus, point a in the circuit diagram is at high voltage (near +5V) and the input signal to the inverter is high. The low output signal (point b) from the inverter creates a voltage drop across the LED causing it to turn on and emit light. In the absence of light, the photodiode has high resistance such that little current flows. Point a in the diagram is at low voltage (near 0V) and the inverter produces high output at point b. The resulting lack of voltage drop across the LED means that the LED is turned off. Much of this exercise will focus on RL and varying the signal between the photodiode and inverter to affect circuit operation.
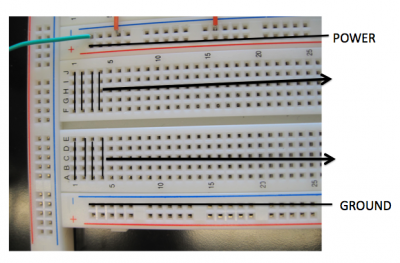
The circuit has been constructed using a breadboard which is a convenient way to construct electrical circuits. The breadboard holes are connected as shown in the photo. Take note of these connections because they'll affect how you will connect up resistors in this exercise.
Examining the extremes of system behavior
A general technique that engineers use to understand a system is they look at the simple, extreme cases and try to reason out the behavior of the circuit. We'll try this to familiarize ourselves with the system. Most of this circuit has been implemented for you. Comparison of the photo with the circuit diagram above should show that the circuit is wired up as shown in the diagram with the exception of the RL resistor. To begin, you'll examine the two extreme cases for the value of the RL resistor: 0Ω and ∞Ω.
Case 1: RL resistor = ∞Ω
Notice that in fact the electronic circuit differs slightly from the original circuit design. There is no RL resistor connecting the photodiode (point a) and ground. Thus, it is as if your circuit has infinite resistance between point a and ground (absolutely no current can flow). Connect the binding posts on the breadboard to the power supply making sure you don't mix up the +5V and the ground. Now turn on the power supply and verify that it is set to +5V. (The display should read 5.00 and the CV light should be on indicating that the power supply is producing constant voltage.)- What happened? Is the LED light on or off?
- Explain why you observe the behavior you do given that there is infinite resistance between the photodiode and ground.
Case 2: RL resistor = 0Ω
Now examine the other extreme case. Connect a wire between ground and the photodiode. Sample connection points are indicated in the circuit photo with circles. Wires tend to have very little resistance so this is almost putting a resistor of 0Ω between the two points.
- What happened? Is the LED light on or off?
- Explain why you observe the behavior you do given that there is almost no resistance between the photodiode and ground.
Construct a functioning circuit
Now that you've explored the two extremes, let's try to actually build a functioning circuit. If you think back to physics, Ohm's law define the relationship between voltage, current and resistance.
$ V=IR $
We can use this formula to calculate loose bounds on RL that will yield a working circuit.
$ \frac{V_{IH}}{Light Current} < R_L < \frac{V_{IL}}{Dark Current} $
- VIL: Highest voltage at point a that is a low input signal for the inverter.
- VIH: Lowest voltage at point a that is a high input signal for the inverter.
- Light Current: Current that flows through the photodiode when light is shone on it.
- Dark Current: Current that flows through the photodiode when it is in the dark.
Conveniently, inverters and photodiodes have been characterized so well that their performance is very well-documented. (Contrast this to parts in the Registry of Standard Biological Parts in which very few parts have any characterization data at all. We have a long way to go!) You should be able to obtain values for VIL and VIH from the inverter datasheet linked below and values for the light and dark current from the photodiode datasheet. These datasheets have far more detail than the sample datasheet for BBa_F2620 shown.
- Determine values (with units!) for the four variables from the datasheet and explain from where on the datasheet you retrieved them. Then calculate the bounds on RL to help narrow your search for a resistor or resistors that will lead to a working circuit.
These bounds will not be exact because the performance of real components deviates some from the model predicted by the equation. Nonetheless, this calculation should give you a place to start for finding an RL that gives a working circuit.
Now, via trial and error, try to find a resistor or series of resistors (resistors connected end to end) for RL that lead to proper circuit operation: in the light, the LED is on and in the dark, the LED is off. Make sure that one resistor end is connected to the photodiode and the other to ground (as shown by the purple circles in the circuit photo). You will need to verify that you are wiring up the resistors properly given the layout of connections on the breadboard! Use the resistor color code table linked below to check that you are using resistors of the resistance you want.
- What resistance value for RL gives a working circuit?
Notice how much easier it is to assemble electrical circuits as compared to biological circuits. It takes seconds to swap in a new resistor into your circuit but a few days to assemble two BioBricks together!
Sensitivity of system function to RL
Next, let's determine how sensitive system performance is to the value of RL.
- How large can RL be and yet have the circuit still work? How small?
You don't need to determine the exact range for RL but getting approximate, experimentally-determined limits gives you an idea of how robust the circuit is to RL. This is analogous to determining how sensitive an engineered biological system is to RBS strength or protein-DNA binding affinity. But again, it is much easier to do this experiment with an electrical circuit.
Comparing resolutions
In fact, the bacterial system does have one important advantage over this electrical circuit. It is much easier to generate lots of copies of a bacteria than lots of copies of your electrical circuit.
- Compare the resolution of a bacterial photograph with that of a photodiode array assembled from many MRD500 photodiodes. What about a typical digital camera on the market these days? Which one has the best resolution? The worst?
Optional
Notice that in fact this electrical circuit behaves opposite the bacterial photography system. The LED readout is on when light shines on the photodiode. In contrast, in the bacterial system, the lacZ readout only occurs for cells in the dark.
- To impress your TA and lab instructor, propose a redesign of this circuit that will yield the same behavior as the bacterial photography system.
DONE!