20.109(S21):Laboratory tour
Contents
- 1 Introduction
- 2 Statement on collaboration and integrity
- 3 Orientation questionnaire
- 4 Laboratory Partner questionnaire
- 5 Interactive and equipment stations
- 6 Benchwork
- 7 Prep notes
Introduction
The main goals for today are to get to know one another and assign laboratory partners. Though the course will be remote this semester, another goal is to familiarize everyone with common techniques and equipment used in scientific research. The Instructors will provide demonstrations for common laboratory tools and information regarding equipment is included below. The next laboratory session will start with an Orientation quiz that is based on the exercises you should complete today.
Statement on collaboration and integrity
Part of becoming a scientist/engineer is learning the ethical conventions of one's discipline. We ask you to rigorously present your results and to cite others' work appropriately not as arbitrary hoops to jump through but as part of your professional training. Integrity in depicting results improves reproducibility and reduces unnecessary frustration in the community who tries to build upon those results. Integrity in citing sources not only gives credit to those who have earned it, but also lets others independently assess the merits of both the original work and the conclusions you have drawn from it.
In 20.109, documentation of your experiments must be completed entirely by you unless otherwise stated. Thus, while we encourage you to discuss your results with your lab partner and other classmates, you may not share text or figures when completing individual assignments, if you are unsure if an assignment should be completed individually or in partners, just ask! Please do not plagiarize -- accidentally or otherwise -- the class wiki or any other writing / images available online or elsewhere. Note that plagiarism is often unintentional, so take the responsibility now to learn the difference between appropriate paraphrasing and academic dishonesty. The following links may help you in this endeavor:
- MIT Libraries overview on citation, especially
- Consequences of academic dishonesty, from MIT Academic Integrity Handbook
Finally, to ensure you to have the most meaningful learning experience possible, and to maintain a fair playing field for all students in the class, we ask that you do not look at completed assignments for similar modules used in previous years.
Orientation questionnaire
Please paste this section into a word editor, fill in the answers, and upload your document to Stellar for the 'Orientation questionnaire' assignment.
- Last name:
- First name:
- Preferred name / nickname (if not your first name):
- Preferred pronouns:
- Course / minor:
- Year of graduation:
- Telephone number (in case we can't find you!):
- Have you taken / are you taking (yes/no + when)?
- 7.05/5.07 (biochemistry)
- 7.06 (cell biology)
- 7.03 (genetics)
- 5.310 (general chemistry lab)
- Do you have experience with... (yes/no + type)?
- Cell culture (microbial/mammalian/yeast?)
- Molecular biology (electrophoresis, PCR, etc)
- Please briefly describe any previous laboratory experience.
- Are you planning on UROP'ing this semester? in which laboratory?
- In which student organizations / varsity teams do you participate?
- What is your favorite hobby these days?
- Do you have any concerns about this semester that you would like to share with the Instructors?
- How is scheduling? Are you in a different time zone? Do you need to share computer access with someone?
- How are resources? Do you have a reliable computer? Is your internet spotty at times?
Please sign your name (with signature image or electronically) under the following statement to indicate your agreement: I have read and understood the 20.109 statement on collaboration and integrity.
Laboratory Partner questionnaire
Please complete the attached document Laboratory Partner Questionnaire and email to Becky (rcmeyer@mit.edu).
Interactive and equipment stations
Station 1: Introduction to pipetting (demonstration)
An Instructor will demonstrate how to use pipetmen for measuring small amounts of liquid. During the demonstration, you should take notes regarding the following information:
- How is liquid siphoned into and expelled from the pipetman?
- What are the ranges of each of the pipetman?
- Are pipetman more accurate at the higher or lower range?
Station 2: Introduction to making solutions (video tutorial)
Making solutions is a fundamental part of being in lab, and the success of your experiments is absolutely dependent on doing it correctly and consistently. For this station, you will watch a tutorial showing best practices to make 40 mL of a 2% (w/v) dextrose solution, linked here: [Station 2]. The steps are included below so you can follow along!
Part 1: Measuring solids
- Put on gloves and eye protection to weigh out solids. This protects you from the chemicals and protects the chemicals from getting contaminated with anything foreign on your hands. Dextrose is a sugar; it is not a dangerous chemical.
- Zero the balance with a weigh boat on it. Weigh boats are kept in the drawer under the balance. The button marked -> O/T <- will zero (“tare”) the balance and the display should read 0.0000 after taring. Be sure to close the balance doors when taring the balance.
- Use a spatula to measure 0.80 grams of dextrose. Open the balance doors and hold the spatula and chemical over the weigh boat. Begin by adding only a small amount of the powder to the weigh boat. Once you determine how much that weighs, you can add correspondingly more. If you have weighed out too much, you can put some back as long as you have used a clean spatula and a clean weigh boat.
- Remove the weigh boat with your dextrose from the balance, gently bend the corners together and pour the contents into a 50 mL conical tube. Tap the back of the weigh boat to loosen any powder that is stuck. The weigh boat can be discarded in the trash since dextrose is not dangerous.
- Clean the balance with a brush. Clean the area around the balance with a damp paper towel.
Part 2: Measuring liquids
You want the solution to have a final volume of 40 mL. Since the dextrose will take up some volume, start with 30 mL of water, add the dextrose, and then adjust the final volume of the solution to 40 mL. This is commonly written as 'adding water up to 40 mL' in protocols.
- Measure approximately 30 mL of distilled water into a 100 mL graduated cylinder. Read the volume in the cylinder by bringing it to eye level to see where the meniscus reaches. Add the water to the 50 mL conical tube containing your dextrose.
- Vortex until all the powder is dissolved.
- Pour the solution back into your graduated cylinder.
- Add distilled water up to 40 mL using a plastic disposable pipet and electronic pipettemen.
- To open the pipet, hold it in one hand; with your other hand, puncture the wrapper by pulling it against the top of the pipet (not the end with the tip!). Put the exposed end of the pipet into an electronic pipette, then withdraw the pipet from the rest of the wrapper.
- Place the tip of the pipet into the distilled water and withdraw enough liquid to “top off” your solution. Dispense the water into your dextrose while submerging the pipet tip in the solution. Stop when the graduated cylinder reads 40 mL. Extra water can be discarded into the sink and the used pipet can be discarded in the "burn box" near the teaching bench.
- Rinse the graduated cylinder with distilled water.
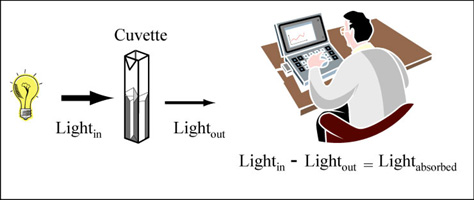
Station 3: Introduction to using a spectrophotometer
A spectrophotometer is used to measure the amount of light lost after passage through an optical component, or sample. The readout from this type measurement provides information regarding the absorbance or optical density of the sample. Though both outputs provide information about the amount of light absorbed by the sample, the interpretation of the measurements is not the same.
Part 1: Measuring absorbance
Color is created when white light strikes a molecule that then reflects light of a certain wavelength and absorbs all the others. A spectrophotometer is an instrument that measures the amount of light absorbed by a sample. It does this by shining light of a particular wavelength into a sample and measuring how much of the light travels through the sample.
There are two important things to remember about measuring absorbance with a spectrophotometer. First, different compounds absorb different wavelengths of light. The color you see is from wavelengths that are reflected by the compound, while the other wavelengths you don't see are absorbed by the compound. For example, red pigments absorb greenish blueish light (light of ~400 nm wavelengths) and blue pigments absorb orangish reddish light (light of ~600 nm wavelengths). Therefore all spectrophotometers have ways of adjusting the wavelength of light shining into the sample. The second important point is that the amount of light absorbed by a sample is directly proportional to the concentration of that sample. This is a very useful relationship defined by Beer's Law. According to Beer’s Law, absorbance is linearly related to concentration by a wavelength- and substance-specific factor called the molar absorptivity (ε).
Part 2: Measuring optical density
Optical density measures the amount of attenuation, or intensity lost, when light passes through a sample. Often optical density is used to measure turbidity in cell cultures, or the density of the cell population. In this, a more dense cell culture (or a culture that has more cells) will result in less light passing through the sample. In the example of measuring cell density, light is largely lost due to scattering.
To determine the optical density (OD) of a culture, the absorbance at 600 nm is measured. This wavelength is used because it is not harmful to the cells in the sample. The OD measurement can then be used to calculate cell number. For E. coli, a common bacteria used in research, an OD600 = 1 corresponds to 8 x 108 cells.
Station 4: Introduction to using a gel documentation system
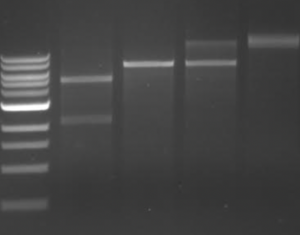
Gel documentation systems are used to photograph agarose gels illuminated with UV light. This is typically used in visualizing DNA products following restriction enzyme digests. We will discuss restriction enzyme digests in much more detail this semester, for now the focus is on how DNA is visualized using dyes and UV light.To image DNA, a dye or stain must be used. DNA dyes are commonly intercalating agents that resemble DNA base pairs. This allows the dye to bind between the bases in a DNA strand. Upon excitation with ultraviolet (UV) light, the dye emits a flourescent signal that can be imaged using a gel documentation system, which consists of a UV table and a camera contained within a box that excludes light. To image DNA that is within an agarose gel, the gel is placed on top of the UV table inside the box. After closing the box to ensure a dark environment, the UV plate is turned on and the camera is used to capture and image of the emitted fluoresecent signal from the gel. As seen in the example to the right, the image appears as a dark background with bright bands. The bright bands indicate the presence of DNA in the gel.
Benchwork
Station 5: Introduction to laboratory safety equipment
Research laboratories are stocked with safety supplies and equipment to ensure the health and well-being of those that conduct experiments within the space. Certain supplies and equipment are common to most laboratory spaces, whereas other items may be necessary when specific types of experiments are conducted in a space. The items below are common and will likely be found in any laboratory on campus:
- Safety showers are used when a researcher spills a hazardous substance on themselves. The safety showers are often located outside of the laboratory space, in the hallway, and do not include drainage as this could promote the release of dangerous chemicals into the environment. If you find yourself in need of a safety shower, you first want to alert a laboratory mate that a spill occurred then remain under the shower for at least 15 minutes. Afterward, seek medical attention.
- Spill kits are used when a research spills a hazardous substance on an innate surface. Spill kits should be stored in a well-labeled location within the laboratory. At MIT, the Environmental Health and Safety (EHS) offices provides spill kits with the following:
- HazMat sock. Socks are crucial for stopping spills from reaching drains or containing the spill. It will also neutralize acids/bases.
- HazMat pillow. A pillow is a condensed absorbent and can absorb a large amount of material and also neutralize acids/bases.
- HazMat spill pads. The spill pads can absorb a lot of material and neutralize acids/bases.
- Nitrile gloves.
- Hazardous Materials marked yellow bag to put compliant used absorbent into for waste disposal.
- Red Tag for waste disposal.
- 1- 5 Gallon Pail that all the material is packaged inside. The pail itself can be used to contain the used absorbent for waste disposal.
- Eyewash stations are used when a research splashes a hazardous substance into their eyes / face. Eyewash stations are often located at the sinks within the laboratory space. If you find yourself in need of an eyewash station, you first want to alert a laboratory mate that a splash occurred then remain at the eyewash for at least 15 minutes. Afterward, seek medical attention.
- Biohazard boxes are used for the disposal of all consumables used in conducting benchwork, particularly consumables that are contaminated with biohazardous materials. At MIT, all materials used in performing experimental work should be disposed of in biohazard boxes as it is assumed that laboratory consumables are contaminated for safety reasons. In this, laboratory supplies should never be discarded in the regular trash.
- Biohazard sharps containers are used for the disposal of sharp objects that are contaminated with biohazardous materials. Sharps include needles, razor blads, and glass pipet tips. As in the case of biohazard boxes, it is assumed that laboratory consumables are contaminated for safety reasons and sharps should never be discarded in the regular trash. Furthermore, due to puncture risks no sharp object (ie broken glass) should be discarded in the regular trash!
- Liquid vacuum flask disposal, or aspirators, are used for the disposal of liquid biohazard waste. Aspirators are typically used for removing spent media from cell cultures and should never be used to dispose of liquids that contain chemicals. Because bleach is often used to clean aspirators, it is very dangerous to use this device for the disposal of liquids that contain chemicals due to the formation of noxious gases.
Station 6: Introduction to data analysis
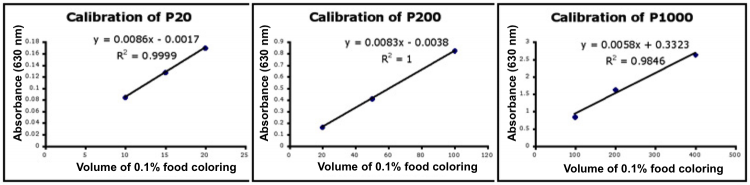
- Use Excel to prepare a graph of absorbance versus volume of 0.1% food coloring using sample data (linked here). Example graphs are provided below and you should generate similar results with the provided data. Be sure to include a trendline, displaying the equation as well as the r-squared value on the graph. The r-squared value reflects how well the data points fit the equation. A perfect fit will give an r-squared value of 1. If you are uncertain how to make such a graph using Excel, be sure to ask for help. We will use Excel a lot this semester.
- Axis labels can be made by going to View → Formatting Palette → Chart Options.
- If the pipets were well calibrated and the measurements were done carefully, then the points should fall close to a straight line, and the r-squared will be close to 1. If one point seems way off, you can re-test that pipetman. If the repeat data still does not look linear, we can clean the inner workings of your pipetman. Note that the highest food coloring concentration may not fall in the linear range of the experiment.
- There should also be good agreement between the 20 μL measurements made with the P20 and the P200 as well as the 100 μL measurements made with the P200 and P1000. Is there?
- Confirm that your data can print from your laptops to the sleaterkinney.mit.edu printer located under the front of the teaching bench. (Note that it can do duplex printing!) You do not need to turn in your printed graphs.
Station 7: Introduction to laboratory math
The information and exercises provided here are intended to refresh your memory of basic lab math concepts. If they are entirely new to you or if you are struggling with the practice problems, please ask for help! It is important that you are comfortable with the information presented here for the Orientation quiz.
Part 1: Metric system
This is the numerical language of science. Base units that you will most often use in this class are meters, grams, liters, and moles. These units will be appended with prefixes to modify the unit by a power of ten.
103 = 1000 = 1000/1 = 103/1 kilo (k-) 100 = 1 = 1/1 = 100/1 base unit (-g, -L, -mol…) 10-3 = 0.001 = 1/1000 = 1/103 milli (m-) 10-6 = 0.000001 = 1/1000000 = 1/106 micro (μ-) 10-9 = 0.000000001 = 1/1000000000 = 1/109 nano (n-)
Practice problems:
- The distance between two cells in 800 μm. How many mm is that?
- The amount of dextrose you want to weigh is 1.9 g. How many mg is that?
- The volume you want to measure is 100 mL. How many liters is that?
- Your reaction generates 0.1 μmoles of product. How many nmoles is that?
Scientific notation expresses numbers so there is one digit to the left of the decimal point and that number is multiplied by a power of ten. 2334 becomes 2.334 x 103 and 0.0041 becomes 4.1 x 10-3. Computations are easier with numbers in scientific notation and some numbers are easier to write (602,214,199,000,000,000,000,000 versus 6.02 x 1023).
Practice problems: Convert the following to scientific notation
- 1000
- 2
- 0.0023
- 0.000000467
The metric system and scientific notation go hand in hand, making unit conversions straightforward. For example 100 μL can be converted to mL by writing the starting volume in scientific notation (1.00 x 102 μL) and multiplying by the power of ten that separates the units (1 mL = 1 x 103 μL). Set up every equation so the units will cancel properly when you multiply through.
Practice problems: Be sure you can express your answers in scientific notation.
- How many mL is 100 μL?
- How many mg is 0.023 g?
- How many mmoles is 250 μmoles?
Note: When using scientific notation in the lab, be sure to keep in mind significant digits. Just because a calculator outputs a value to the seventh decimal point, doesn't mean that you actually know that value to such precision! Throughout the semester, please be thoughtful when working with numbers obtained both from direct measurements and from calculations.
Part 2: Concentrations
Molarity (moles/liter) is a common expression of concentration. When making a solution of a particular molarity, you need to know three things: the desired molarity, the desired volume and the formula weight of the compound to be dissolved. The best place to find the formula weight (grams/mole) is on the chemical’s bottle. Calculations are performed by setting up an equation so that the units cancel, leaving grams in the numerator and volume in the denominator.
Another common expression of concentration is percent. Percent solutions are always based on 100 mL. For powdered substances, percent solutions reflect the weight (in grams) in a 100 mL volume (“w/v”). For example, a 10% solution of NaCl is 10 grams in 100 mL of water. In fact a 10% solution of any powdery substance is 10 grams in 100 mL. For liquids, percent solutions reflect the volume in a 100 mL final volume (“v/v”). For example, a 70% ethanol solution is 70 mL of 100% ethanol and 30 mL of water. (Remembering that 1 mL of water weighs 1 gram may help you remember the w/v expression.)
Practice problems:
- You want to make 100 mL of a 0.5 M dextrose solution. The formula weight of the substance you want to dissolve is 180.16 (g/mol). How many grams will you measure?
- You want to make 10 mL of a 0.01% (w/v) solution of a blue dye called Xylene Cyanol. How many grams will you dissolve?
- How would you make 100 mL of a single aqueous solution that is 5% (v/v) acetic acid and 5% methanol?
Part 3: Dilutions
Many solutions are made by diluting concentrated stock solutions. Dilution factors of 1:2, 1:5, 1:10 and 1:100 are common. These dilutions are made by diluting one “part” stock with 1, 4, 9 or 99 “parts” water, respectively. For example, you could make 100 mL of a 0.5 M dextrose solution by mixing 10 mL of a 5 M stock solution with 90 mL of water. This is a 1:10 dilution of the stock. The dilution factor can be converted to a fraction to determine the solution’s final concentration (5 M x 1/10 = 0.5 M).
When the dilution factor is less obvious, the formula C1V1 = C2V2 can be used, where C1 is the starting concentration of the stock solution, C2 is the desired concentration, V1 is the volume of stock you’ll need (usually this is your unknown) and V2 is the final volume you want to make. For example, to make 1000 mL of a 0.2 M Tris from a 1.5 M stock you would multiply 1.5 M (V1) = 0.2 M (1000) to find that you will need 133 mL of the stock. To determine how much water to add you would subtract V2 – V1, in this case 1000 mL – 133 mL = 867 mL of water.
When solutions must be diluted several orders of magnitude, then serial dilutions are made. The concentrated stock is progressively diluted, for example using a 1:100 dilution as the new “stock” in another 1:100 dilution. Such a serial dilution produces a solution that is 10,000 times less concentrated than the starting material. One benefit to serial dilutions is that small volumes of each dilution can be made accurately. A drawback is that any pipetting or calculation error is propagated through every dilution.
Practice problems:
- How would you make 50 mL of a 1:5 dilution?
- Give the volume of stock and the volume of water necessary to make 50 mL of a 0.25 M solution starting with a 2 M solution.
- A concentrated culture of bacteria has approximately 1 x 108 cells/mL. What is the concentration of bacteria after it has been diluted 1:100? What is the concentration of bacteria if a 1:2 dilution was made of the 1:100?
Station 8: Introduction to course wiki
The great thing about the wiki is that everything is on the wiki, and the bad thing about the wiki is that everything is on the wiki. To help you familiarize yourself with navigating the most important resource in 20.109, answer the following questions as you explore the wiki tabs. To make navigation easier, the toolbar is located within the header at the top of every page and contains links to the tabs you will use most frequently this semester.
- Click the FYI link in the navigation toolbar at the top of this page. This tab contains information on the Teaching Team, including contact information and office hour times. The attendance policy for the course is also included here. What will result if you have an unexcused absence from the laboratory?
- Click the Assignments link. This tab includes information on how your grade will be determined. What is the policy for submitting late work (specifically, how are late assignments graded)?
- Click the Homework link. All of the homework assignments are listed here and organized by the day due. What information is included in the 'Wrapping up...' sub-section?
- Click the Communication link. The Communication Lab Instructors will provide five workshops this semester to assist you in preparing your major assignments. What will you learn in the first workshop (the link is broken, but you can make an assumption based on the title of the workshop)?
- Click the links associated with each of the modules and briefly review the information on each tab. What type of information is linked to these tabs?
Next, the Schedule tab is the most valuable page and the teaching faculty highly recommend that you bookmark it!
- The lecture slides are posted to the Schedule tab. Where are the Orientation lecture slides posted?
- The prelab slides are also posted to the Schedule tab. Where are the prelab slides for your laboratory section posted?
- What information is linked to the Laboratory Experiments column?
- What information is linked to the Assignments column?
Lastly, you may have noticed that information is linked to several pages (where it is relevant) for ease of navigation.
- List all of the pages you can find that contain a link to the M1 Data summary page. Provide at least three!
Station 9: Introduction to tissue culture facility
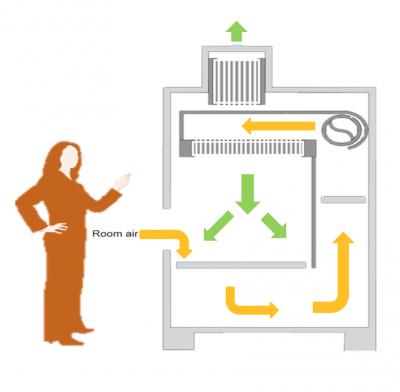
To culture mammalian cells, specialized supplies and equipment are required. Later in the semester we will discuss the specific types of culture vessels and tools that are used. For now, please review the information regarding the important equipment below:- Biosafety cabinets, or cell culture hoods, are used to maintain a sterile environment when working with cell cultures. As depicted in the image to the right, room air is pulled into the biosafety cabinet and circulated through ducts around the work space (shown by yellow arrows). The room air is then passed through a hepa filter to remove debris and contaminants. The sterile air is blown from the top of the biosafety cabinet onto the center of the work space (shown by green arrows in the center of the image). For this reason, it is best practice to work in the center of the work space of the biosafety cabinet. Sterile air is also released into the environment (shown by green arrow at the top of the image).
- Cell culture incubators are used to promote the growth of mammalian cells. In general incubators are used to maintain a specific temperature. For cell culture incubators, temperature is maintained at 37 °C, or body temperature. In addition to temperature, humidity and carbon dioxide are maintained at 95% and 5%, respectively. Carbon dioxide is used to stabilize the pH of the cell cultures and humidity is important in preventing evaporation from the cell culture flasks.