20.109(F19):Complete antibody staining for Western blot analysis (Day4)
Contents
Introduction
Traditionally, only one species of antibody could be used on a Western blot because the detection relied on the emission of light that was collected by x-ray film. In the traditional systems the output looks like black bands on a blue or clear background. However, more recent conjugate chemistry has allowed secondary antibodies to be coupled to fluorescent tags. Today we will use infrared (IR) secondary antibodies to detect our α-FKBP12 and α-tubulin antibodies and then scan the Western blots using a specially constructed microscope located in the Lauffenburger lab.
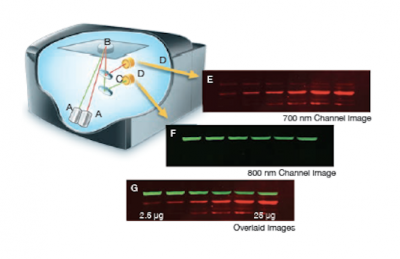
The Licor Odyssey scanner consists of an inverted microscope with two lasers that excite dyes which emit light in the IR range. As depicted in the image on the right, an excitation point is created when beams from the 700 nm and 800 nm lasers (A) are focused on the scanning surface. The microscope objective (B) is focused on the excitation point and collects light from the fluorescing IR dyes. This light is passed through a dichroic mirror (C) that separates the light into two distinct signals that travel through two independent optical paths that are focused on separate silicon photodiodes (D) and detected. In the image, the first channel (E) and second channel (F) are shown separately and merged (G).We will detect our IR-dye conjugated secondary antibodies at wavelengths of 700 and 800 nm. The 700 nm channel will appear red and the 800 nm channel will appear green. Infrared secondary antibodies provide a more flexible detection platform than the traditional Western blot detection methods that rely on colorimetric or chemiluminescent substrates. Unlike the colorimetric or chemiluminescent detection methods, IR dyes do not require a chemical reaction to occur in order for signal to be detected. This means that the output signal increases with time as the colorimetric or chemiluminescent substrate reaction proceeds -- making timing an important variable in traditional Western blot development. We remove that variable from the equation and control when we want to visualize our Western blot simply by controlling the excitation of the dye.
Protocols
The blot that you prepared in the previous laboratory session was stored in the 4 °C cooler in blocking buffer.
Part 1: Apply primary antibody
- Retrieve your blot from the front laboratory bench.
- Prepare the primary antibody solution.
- Dilute the primary antibodies in 10 mL of blocking buffer.
- For the FKBP12 you will use rabbit anti-FKBP12 antibody at a 1:1000 dilution.
- For the α-tubulin you will use mouse anti-α-tubulin antibody at a 1:5,000 dilution.
- Pour off the blocking solution in the sink.
- Add the primary antibody solution to your blot.
- Carefully place the blot on the rotating table and cover with aluminum foil.
- Shake at 65 rpm for 60 min.
Part 2: Apply secondary antibody
- Obtain your blot from the rotatating table and pour the primary antibody solution into a conical tube.
- Label with team and section information.
- Because the antibody is in excess, the diluted primary solution can be re-used and is worth saving until you see the results of your Western blot.
- Add enough TBS-T to cover the membrane.
- Return your blot to the rotating table for 5 min at 80 rpm.
- Repeat for a total of 3 washes.
- Immediately before pouring off the last wash, prepare the secondary antibody solution.
- Dilute the secondary antibodies in 10 mL of blocking buffer.
- For the FKBP12 (raised in a rabbit) you will use the donkey anti-rabbit IR680 (RED) antibody at a 1:10,000 dilution.
- For the α-tubulin (raised in a mouse) you will use the goat anti-mouse IR800 (GREEN) antibody at a 1:10,000 dilution.
- The secondary antibodies are light sensitive and should be kept in the dark! Use aluminum foil to wrap your tube.
- Add the secondary antibody solution to your blot.
- Carefully place the blot on the rotating table and cover with aluminum foil.
- Shake at 65 rpm for 60 min.
- Pour off the secondary antibody in the sink.
- Wash the membrane by adding TBS-T and shake for 5 min at 80 rpm, using the rotating table.
- Repeat for a total of 3 washes.
- The Odyssey scanner is located in the Lauffenburger laboratory, one of the teaching faculty will accompany you there to scan your blot.
Part 3: Discuss research proposals with peers
To help you focus your ideas and develop the details of your project, you will discuss the project description you submitted today with a classmate from another group. As you listen to your classmate's idea, consider the following criteria proposed for small research project grants by the NIH:
Small Research Grant Program: ...small grant supports discrete, well-defined projects that realistically can be completed in two years and that require limited levels of funding. Because the research project usually is limited, the grant application may not contain extensive detail or discussion. Accordingly, reviewers should evaluate the conceptual framework and general approach to the problem. Appropriate justification for the proposed work can be provided through literature citations, data from other sources, or from investigator-generated data. Preliminary data are not required, particularly in application proposing pilot or feasibility studies.
Use the following exercise to guide your discussion as you consider both your and your classmate's project. Because you are still in the early stages of developing your research topic, it is okay if you do not have all of the answers to the following questions. This is meant to help you critically think about your proposal...not to point out the additional research you need to complete! Furthermore, this is an informal conversation and you should feel free to look up information during this exercise or just make notes so you know what to research later with your co-investigator.
Outline of the peer review exercise:
- Find the partner you were assigned by the teaching faculty and begin by deciding which partner will present first.
- As the presenter, focus on why you believe your topic is important and provide the context needed to convince your listener that it is indeed worth pursuing.
- As the listener, verbally summarize the topic back to the presenter to ensure you understood the proposal. Are you convinced that the topic is important? Why or why not? Discuss this with the presenter.
- Now that you have the needed background information discuss the 'Hows' of the project.
- As the presenter, consider the questions below as you give some details about your proposal.
- As the listener, feel free to ask questions and maybe provide some helpful feedback as the presenter discusses the details of their project.
Questions to guide your discussion:
- What is the novel aspect of your proposal?
- Why do you believe your project is feasible?
- Is there evidence that supports your proposal?
- How will your research advance the field?
- How will you complete your research (what methods might you use)?
- What is the expected result?
- How might you 'double-check' or confirm an expected result?
- What if you do not get the expected result?
- What can be learned if you get the expected result? If you get an unexpected result?
- What are some alternative approaches (methods) for your proposed research?
Once you have completed discussing the presenter's project, switch roles and complete this exercise with the listener's project.
Use the information you gathered during this exercise to drive a discussion with your co-investigator as you further develop your research proposal.
Reagents list
- Odyssey blocking buffer (from Licor)
- FKBP12 primary antibody (from Abcam)
- α-tubulin primary antibody (from Proteintech)
- donkey anti-rabbit IR680 secondary antibody (from Licor)
- goat anti-mouse IR800 secondary antibody (from Licor)
Next day: Data analysis and assignment preparation