Difference between revisions of "Lab Manual: Optical Microscopy"
(→White light microscope) |
(→=Bright field transmitted illumination imaging) |
||
| Line 116: | Line 116: | ||
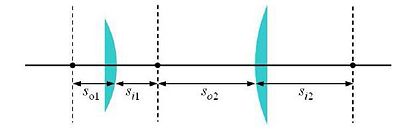
[[Image:20.309Hw3Image4flens.JPG|center|thumb|400px|Figure 4: A 4-f lens system using lenses with focal lengths f1 and f2. The object and image distances (so and si, respectively) for the lenses are indicated.]] | [[Image:20.309Hw3Image4flens.JPG|center|thumb|400px|Figure 4: A 4-f lens system using lenses with focal lengths f1 and f2. The object and image distances (so and si, respectively) for the lenses are indicated.]] | ||
| − | ===Bright field transmitted illumination imaging== | + | ===Bright field transmitted illumination imaging=== |
Sketch out a rough design for your microscope on paper. Begin with the bright field illumination path. | Sketch out a rough design for your microscope on paper. Begin with the bright field illumination path. | ||
| Line 128: | Line 128: | ||
* The barrier filter has been installed in a lens tube already attached to the camera. | * The barrier filter has been installed in a lens tube already attached to the camera. | ||
| − | ===Fluorescence imaging | + | ===Fluorescence imaging=== |
Now sketch a layout that includes both the bright field and fluorescence illumination paths. | Now sketch a layout that includes both the bright field and fluorescence illumination paths. | ||
Revision as of 03:03, 27 October 2008
Contents
- 1 Introduction
- 2 Objectives
- 3 Roadmap and Milestones
- 4 Microscope Construction
- 5 Microscope design
- 6 4 Experiment 1: Microscope Characterization and Fourier-Plane Imaging
- 7 5 Experiment 2: Microrheology Measurements by Particle Tracking
- 8 6 Experiment 3: Fluorescence Imaging of the Actin Cytoskeleton
- 9 7 Report Requirements
Introduction
In this lab, you will design and build a light microscope from optical components. Your instrument will be capable of two kinds of imaging: bright field transmitted light and fluorescence. In bright field,
Bright field transmitted microscopy is perhaps the simplest and most common optical microscopy method. In this technique, photons from an illuminator pass through the sample, where the may be absorbed, diffracted, or refracted. (The sample us usually mounted on a glass slide.) An objective lens on the opposite side of the sample collects the light. Most modern objective lenses produce collimated light, which is focused by a tube lens to form an image.
Illumination for fluorescence microscopy normally reaches the sample through the objective lens — from the same side of the sample that is observed. Fluorescence microscopy is normally used on samples that have been labled with a fluorescent molecule called a fluorophore. The (narrowband) illumination wavelength must match the absorption characteristic of the fluorophore. After becoming excited by a photon from the illuminator, the fluorescent molecule will emit a photon of longer wavelength. A dichroic mirror in the microscope reflects the illumination wavelength but allows the emitted photons to pass through.
The microscope design in thie lab is very flexible. Other contrast modes, such as dark field illumination and confocal, can be added to the microscope.
Objectives
- Learn about the theory and practice of light microscopy
- Use ray tracing rules to design a transmitted bright field and fluorescent light microscope
- Construct the microscope from optical components
- Characterize the microscope's performance
- Enhance and analyze microscope images
- Quantitatively track moving particles in the field of view
Roadmap and Milestones
Week 1
- Design and Build a microscope
- Characterize the transmitted bright field performance of the microscope
- Add a laser illumination beam path
Week 2
- Characterize the fluorescent imaging performance of the microscope
- Make fluorescent images
- Correct for nonuniform illumination
- Track microspheres suspended in a solvent
- Estimated diffusion coefficients
- Track the motion of vesicles under transport in a plant cell
Microscope Construction
An example microscope made by the instructors will be available in the lab for you to look at. Feel free to make improvements on this design. Stability will be crucial for the particle tracking experiments. This will be achieved through good design and careful construction — not by mindlessly overtightening screws.
Microscopy lab etiquette
- Observe all laser safety guidelines.
- Keep all of the boxes for the optics you use with your instrument to simplify putting things away.
- The stages are very expensive. To prevent accidents, ensure that there is a srew holding the post base to an optical breadboard or table at all times.
- There are not enough stages to go around. Remove the stage from your microscope and leave it at the lab station when you are done.
- Leave the illuminator at the lab station when you are done.
- Return objective lenses to the drawer when you are not using them.
- Never use an SM1T2 coupler without a locking ring — they are very difficult to remove if they are tightened against a lens tube or tube ring.
- Use tube rings (never an SM1T2) to mount an optic in a lens tube.
Microscope parts
Rigid optical construction
The design uses a combination to cage and lens tube components from ThorLabs. (See the ThorLabs online catalog for more details. Print catalogs are available in the lab.) Be sure you understand how to use cage cubes (C4W), cube optic mounts (B5C), and kinematic mounting plates (B4C). Please ask about any components you are not sure how to use.
Simple lenses
Plano-convex spherical lenses are available with focal lengths of 25, 50, 75, 100, 125, and 200 mm. It is best to mount most optics in short (0.5" or 0.3") lens tubes. It is acceptable to mount a lens between the end of a tube and a tube ring or between two tube rings. In most cases, the convex side of the lens faces toward the collimated beam; the planar side goes toward the convergent rays. Plano-concave lenses with focal lengths of -35 and -50 are also available.
- Advice: Some students in the past have had difficulty with the Three of These Things game. Verify all optics before you use them by measuring the focal length with a ruler.
As you install lenses into your microscope, put a piece of tape on the lens tube showing focal length and orientation. This will help you both during constructino and put-away. Save the lens storage boxes and return components to the correct boxes when you are done.
Handle lenses only by the edges. If a lens is dirty, first remove grit with a blast of clean air or CO2. Clean the lens by wiping with a folded piece of lens paper wetted with a drop of methanol. (Do not touch the part of the tissue you use for cleaning with your fingers.) In some cases, it may be helpful to hold the folded lens tissue in a hemostat. Ask an instructor if you need help.
Objective lenses
Please see the Nikon Introduction to Microscope Objectives at their excellent MicroscopyU website.
There are three objective lenses available in the lab: a 10×, a 40×, and a 100×. All of these are designed for a 200 mm tube lens. An adapter ring converts the objective mounting threads to the SM1 threads used by the lens tube system.
- The back focal plane (BFP) of the objective coincides with the rear of the objective housing. This is equivalent to the focal plane of a simple lens.
- Working distance (WD) is the distance between the front end of the objective and the sample plane (when
the sample is in focus). Generally, the higher the magnification, the lower the working distance.
- The 100× objective is designed to be used with immersion oil, which provides an optical medium of pre-
determined refractive index (n = 1.5). When using the 100× objective, place a drop of oil on it. Bring the drop in con- tact with the slide cover glass. After use, clean off excess oil by wicking it away with lens paper.
Sample stage
Precision Newport X/Y/Z stages mounted on a post are available at each lab station. The stage setup is top-heavy. Avoid accidents by ensuring that the post base is always attached to an optical breadboard or table. Return the stage to the lab station when you are done with it.
The z-axis adjustment of the sample stage provides fine focusing.
Fluorescence illumination
The fluorescent illumination source is a 5 mW, λ=532 nm green laser pointer. Do not begin working with the laseruntil you are familiar with laser safety procedures. If you missed laser safety training, please see an instructor.
- Wear the provided safety eyewear at all times — even when your laser is not on — since other groups may be using theirs.
- Make sure the laser warning light outside the main door to the lab is flashing before you turn on a laser.
- Never point the laser toward other people.
- Laser light can emerge from the top of the objective lens. Never put your face directly above the objective.
Beam expansion
Collimated light comes out of the laser pointer. The light should also be collimated when it reaches the sample (Kohler illumination). In order to provide a good image, the laser light should illuminate most of the field of view. The beam emerging from the laser pointer (abuot 1.1 mm) may not be wide enough. This means that the laser beam will need to be expanded. What expansion factor should you use for a 40× objective? You may want to be a bit conservative with beam expansion, since the laser pointer is not very powerful. Overexpansion will decrease the light intensity at the sample and may not give you enough power for imaging.
Dichroic mirror and barrier filter
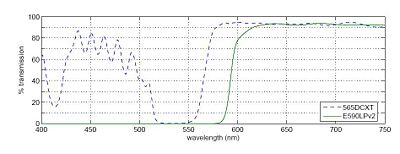
The fluorophores we will emit light in the orange-red region of the visible spectrum (550-600 nm) when excited by the 532 nm green laser.
In any fluorescence system, a key concern is viewing only the emitted fluorescence photons, and eliminating any background light, especially from the illumination source. Two optical elements address the problem. A dichroic mirror passes light of one wavelength, and reflects light of another. The transmission spectrum for the 565DCXT dichroicis shown in Fig. 3. A barrier filter blocks a particular spectral region. We will use the E590LPv2 barrier filter.
Figure 1 shows the tranmittance of the dichroic mirror and barrier filter over a range of wavelengths. The barrier filter is essential for high-sensitivity fluorescence imaging. It will pass the red light from the LED in the illuminator for bright field imaging as well as the fluorescence photons.
CCD camera
The microscope you will build does not have an eyepiece for direct visual observation. Instead, images will be captured with a Firewire-enabled CCD camera (DMK 21F04 from The Imaging Source). Its monochrome (black and white) sensor contains a grid of 640×480 square pixels that measure 5.6 μm on a side. An adapter ring converts the C-mount thread on the camera to SM1.
The barrier filter, SM1 adapter, and a quick-connect have been installed on the camera. Please leave the camera at the lab station when you are done with it. Camera software called IC Capture is installed on the labstation PCs.
Microscope design
A basic microscope is essentially a 4-f system (sketched in Fig. 4). Some elements must be positioned precise distances apart; other distances are not critical. Use ray-tracing to determine when this is the case.
Bright field transmitted illumination imaging
Sketch out a rough design for your microscope on paper. Begin with the bright field illumination path.
Note:
- The Nikon objective lenses are designed to be paired with a 200 mm tube lens.
- Assume that the objectives behave as ideal plano-convex lenses.
- Fine focusing will be achieved by adjusting the height of the sample stage.
- Start the alignment with a 10× objective but progress to 40× and 100×.
- Use the LED illuminators for bright field transmitted light imaging.
- Put a quick connect in your design such that the camera CCD will end up 200mm from the back focal plane of the objective. Remember that the CCD is recessed inside the opening of the camers.
- The barrier filter has been installed in a lens tube already attached to the camera.
Fluorescence imaging
Now sketch a layout that includes both the bright field and fluorescence illumination paths.
- Use a dichroic to direct the laser illumination toward the sample and to pass the emitted (fluorescent) light back through to the CCD.
- The barrier filter removes any light from the illumination laser that was not reflected by the dichroic.
- During fluorescent imaging, you will not use the bright field illuminator. Bright field capability is useful for first visualizing the sample and viewing what features are in the field of view. Your design should retain the ability to do both white light and fluorescent visualization.
- Verify your design with one of the lab instructors before beginning construction.
The microscope should be built in two stages. First, build a white-light inverted microscope. Verify its alignment and magnification. Next, add the fluorescence branch.
- An adjustable iris aperture and some lens or tissue paper can be very helpful for aligning the laser and directing it along the axis of the tubes.
4 Experiment 1: Microscope Characterization and Fourier-Plane Imaging
4.1 White light calibration
Use this white light microscope to image the following samples using the three different objectives (10×, 40×, and 100×)
- The smallest line pair on the so-called Air Force imaging target test pattern.
- A slide of 4 μm latex spheres.
- Ronchi ruling - a periodic pattern containing 600 line-pairs per mm. (only use 40× and 100× objectives for this; why?)
Can you see all these samples? What is the magnification of the microscope and the size of its field of view? Is it what you expected?
4.2 Fluorescence characterization and imaging
You have the following samples available for imaging using both 40× and 100× objectives:
(a) A solution slide of Rhodamine 6G (Rh6G) solution, used to adjust the microscope for uniform illumination (this has its excitation peak at 530nm, and a fairly broad band of emission above 550nm).
(b) A sample slide with 4 μm red-fluorescent beads (modified with Nile Red dye, peak excitation at 535nm, peak emission at 575nm).
Imaging tasks:
1. Use the Rh6G slide to optimize the uniformity of the illumination field (you want the light distribution hitting the sample to be as uniform as possible). Take an image of this.
2. Measure the signal level while imaging Rh6G - try to achieve maximal uniformity and brightness.
3. Image the red beads slide. Perform flat field correction on the beads (i.e. divide the bead image by the normalized Rh6G image). Compare what you see before and after flat field correction.
If the images are noticeably different, especially at 40× (i.e. there is significant non-uniformity in the illumination field), you should improve the scope's alignment until there isn't much difference.
4.3 Fourier optics
Recall from lecture that a lens behaves as a Fourier transformer. When an object is placed at a plane one focal length away from the lens, an image of the object's spatial frequencies is formed one focal length away on the other side (called the Fourier plane). Likewise a Fourier transform projected through a lens generates the corresponding image (see Figure 5).
4.3.1 The light-scattering microscope
Here we'll investigate the Fourier transforming properties of our microscope. This capability is employed in a type of microscopy called Dynamic Light Scattering (DLS), which can be used, for example, to quantify the presence of regular patterns or measure average sizes of features being imaged.
To modify your microscope for imaging the Fourier plane, simply add a lens at the plane one focal length behind the image plane and position the CCD camera one focal length behind the lens. (A 50 mm lens is good { it will keep the beam path short, thought the exact focal length is not critical.) Here, a pair of quick-connects will play a key role, letting you quickly switch between capturing the image plane and the Fourier plane on the CCD.
| Figure 5: A lens system with unity magnification,
showing the relative locations of image and fourier planes. |
To characterize the Fourier-imaging ability of your microscope, we'll use the Ronchi ruling, illuminated by the laser. Its spatial frequency components can be readily imaged at the Fourier plane. Before doing the experiment, estimate how many diffraction orders you can observe with the 40£ and 100£ objectives (which have NA = 0:65 and NA = 1:3 respectively)? The general equation describing the optical geometry of a di®raction grating is a(sin µm ¡ sin µi) = m¸ ; where µi (the incident beam angle) and µm (the di®raction angle) are measured with respect to the normal, a is the grating period, m is the di®raction order number, and ¸ is the light wavelength. What is the incident angle of your experiment? What is the maximum di®raction angle allowed by your apparatus? How is µm related to the numerical aperture of the objective lens? Recall that NA = n sin µ, where n is the index of refraction of the medium. For air n = 1 and for oil n = 1:5. Now, view the Fourier plane images of the Ronchi ruling with the 40£ and 100£ objective lenses to verify your calculations.
| Figure 6: [Images and caption reproduced from Vukusic and Sambles] (a) Real color image of the blue
iridescence from a Morpho rhetenor wing. (b) Transmission electron micrograph (TEM) images showing wing-scale cross-sections of M. rhetenor. (c) TEM images of a wing-scale cross-section of the related species M. didius reveal its discretely con¯gured multilayer. The high occupancy and high layer number of M. rhetenor in b creates an intense re°ectivity that contrasts with the more di®usely colored appearance of M. didius, in which an overlying second layer of scales e®ects strong di®raction. Scale bars: (a) 1 cm, (b) 1.8 ¹m, (c) 1.3 ¹m. (d) Blue iridescence is prevalent in the fern-like tropical understory plants of the genus Selaginella. (e) TEM section of a juvenile leaf from the plant Diplazium tomentosum. |
4.3.2 Bio-photonic crystals
Many biological systems in nature have interesting optical properties (please see the fascinating review1 recently published in Nature). Some researchers have advocated using biological system to \grow photonic crystals. Today, photonic crystals made by microlithography have been used to guide light in optoelectronic systems and for optical computing. If photonic crystals can be self-assembled using biological systems, this may become a new low-cost and high-throughput manufacturing approach.
You are provided with two samples. (1) A peacock feather and (2) a piece of tissue paper. Obtain the di®raction pattern from both samples using the 100£ objective. Now remove the lens that generates the Fourier plane, and obtain a real image of both specimens. Can you explain the di®raction patterns observed (quantitatively)? Do either of these samples exhibit the properties of photonic crystals?
If this subject interests you, take a look at the additional references listed in the footnotes.2,3
5 Experiment 2: Microrheology Measurements by Particle Tracking
5.1 Introduction and background
Many cellular functions such as migration, differentiation, and proliferation are regulated by the mechanical properties of cells, specifically, their elasticity and viscosity. Rheology is the science of measuring materials' mechanical properties. Microrheology is a subgroup of techniques that are capable of measuring mechanical property from microscopic material volumes. Clearly, given the typical size of biological cells, microrheology is the technique needed to measure their elasticity and viscosity.
The elastic and viscous properties of cells can be characterized by a complex-valued shear modulus (with units of Pa) G*(w) = G'(w) + iG(w). The real part G'(w), referred to as the storage modulus, is a measure of cell elasticity, while the imaginary part G(w), the loss modulus, is a measure of their viscosity. A generalized Hookian relationship can be written as
F(w) math /propto /math G¤(!)¢r(!) ;
where ¢r(!) is a generalized displacement, and F(!) is a force linearly proportional to it via the shear modulus. Therefore, we can measure the shear modulus if we can measure the deformation of the cell under a known force. (Note that all these quantities are frequency-dependent). Particle-tracking microrheometry is based on measuring the displacement of a particle with radius a embedded in a cell driven by thermal forces (similarly to the vibrations of the cantilever that you observed in the AFM lab). One complication is that this relationship is frequency dependent { this is because in complex °uids, such as the cellular cytoskeleton, there are di®erent energy dissipation mechanisms over di®erent time scales.
To approach the derivation of the relevant formulas, it is more convenient to think in terms of energy, rather than force. The relationship between stored energy and displacement has a familiar form, similar to a spring-mass system (recall KE / k(¢z)2):
U(!) = Z F(!)dr / G¤(w)¢r2(!) :
What is the driving thermal energy U(!)? Recall also from that thermal energy is \white, i.e., it contains equal power at all frequencies and is equal to 1 2kBT for each degree of freedom in a second-order system, where kB is Boltzmann's constant and T is the absolute temperature. From this relationship (since we're observing motion in two dimensions), we have
G¤(!) / kBT ¢r2(!) :
Our argument is clearly very rough but a complete (and much more di±cult) derivation results in the following equation (see Mason4 for details):
jG¤(!)j = 2kBT 3¼a h¢r2(!)i¡[1 + ®(!)]
Some key additional details to help you make sense of this equation: 1. As you can see, the dependence on displacement is more accurately expressed as the mean- square displacement (MSD) h¢r2(!)i: MSD = h¢r2(!)i = h¢r2( 2¼ ¿ )i = h[r(t + ¿ ) ¡ r(t)]2i = 1 N NX i=1 [r(ti + ¿ ) ¡ r(ti)]2 ; where h i denotes a time-average of the particle's displacement trajectory r(t), at discrete times t = t1; : : : ; tn (as sampled by a digital system like the PC and camera). Additionally, ¿ is a characteristic lag/delay time for the measurement. corresponding to the frequency !. 2. ®(!) ´ @ lnh¢r2(¿ )i @¿ ¯¯¯¯¯¿=2¼=!
3. The radius of the particle a plays a role in the formula. 4. ¡(¢) is the Gamma function (the generalized form of the factorial function, which can be looked up in a mathematical table). Mason suggests that for our range of ®, ¡[1 + ®] ¼ ¡0:457(1 + ®)2 ¡ 1:36(1 + ®) + 1:90: This equation may look complicated but there is a simple approximation to calculate the elastic and viscous moduli: G0(!) = jG¤(!)j cos[¼®(!)=2] G00(!) = jG¤(!)j sin[¼®(!)=2] A detailed discussion of particle tracking microrheology can be found in the papers by Mason and Lau5.
5.2 Experimental details
5.2.1 Stability and setup
The major challenge of particle tracking microrheometery is the small scale of the thermal forces and the associated nanometer scale displacements. A few things you can do to ensure the experiment works: Be sure all the microscope components are rigidly assembled and ¯rmly tightened. Poorly built scopes shake. It is also vital that when you perform this experiment that the optical tables are °oating so that building noise is isolated. Of course, avoid touching the optical table and the microscope during the measurement. There are also cardboard boxes available that you can put over your microscope to isolate it from air currents. Finally, make sure that you and the people around you are not talking too loudly during the experiment, because acoustic noise is signi¯cant. The camera gain and brightness setting should be set as described in Figure 7.
5.2.2 System veri¯cation
To verify that your system is su±ciently rigid/stable, ¯rst measure a specimen containing 1 m red °uorescent beads (Molecular Probes) dried in a cell dish. Chose a ¯eld of view in which you can see at least 3-4 beads. Using a 40£ objective record an .avi movie for about 3 min. at a frame rate of 30 frames/sec. From your experience with image processing, you already know how to import .avi movie data into matlab. To improve signal to noise ratio, sum every 30 frames together, which will make your sequence have a temporal interval of 1 sec. Use the bead tracking processing algorithm on two beads to calculate two trajectories. To further reduce common-mode motion from room vibrations, calculate the di®erential trajectory from the individual trajectories of these two beads. Calculate the MSD h¢r2i from this di®erential trajectory. Your MSD should start out less than 10 nm2 at ¿ = 1 sec. and still be less than 100 nm2 for ¿ = 180 sec. If you don't get this, do not proceed further and ask for help. 5.2.3 Live cell measurements Now that your system is su±ciently stable, you can run the experiment on cell samples. A key technique to keep in mind when working with live cells { to avoid shocking them with \cold at 20±C, be sure that any solutions you add are pre-warmed to 37±C. We will keep a warm-water bath running on the hotplate for this purpose, in which we will keep the various media. You are provided with NIH 3T3 ibroblasts, which were prepared as follows: Cells were cultured at 37±C in 5% CO2 in standard 100 mm £ 20 mm cell culture dishes
| Figure7: Proper camera gain settings for particle tracking: Choose Properties... under the Device menu and set the gain and brightness level to zero. Change the exposure time until the intensity of the fluorescent beads is just high enough to achieve good contrast, but do not saturate the intensity value to 255 |
(Corning) in a medium referred to as DMEM++ { this consists of DMEM (Cellgro) supplemented with 10% fetal bovine serum (FBS - from Invitrogen) and 1% penicillin-streptomycin (Invitrogen). The day prior to the microrheology experiments, ¯broblasts were plated on 35 mm glass-bottom cell culture dishes (MatTek). On the day of the experiments, the cell con°uency should reach about 60%. 1 ¹m diameter orange °uorescent microspheres (Molecular Probes) were mixed with the growth medium (at a concentration of 5 £ 105 beads/mL) and added to the plated cells for a period of 12 to 24 hours for bead endocytosis. Choose cells with 3 or 4 particles embedded in them and take a movie as before. Take movies of about 3-5 cells. Now treat the cell with the cytoskeleton-modifying chemical cytochalasin D (CytoD). Pipet out the bu®er, add 1 mL CytoD solution at 10 ¹M (pre-mixed for you) to the dish, and wait for 20 min. It's a good idea to check on your cells after 20 min.: sometimes they are in bad shape at that point but sometimes they still look very healthy. Wash and replace with bu®er twice with 2 mL pre-warmed DMEM++. Repeat the particle tracking measurements again for 3-5 cells as quickly as you are able, since their physiology has now been signi¯cantly disrupted and they will die within a couple of hours. It's very unlikely that you'll be able to ¯nd the exact same cells you've already tracked; however it's very much advisable to use the same dish for the \before and \after so you're aren't also comparing between di®erent cell populations.
6 Experiment 3: Fluorescence Imaging of the Actin Cytoskeleton
We have now observed CytoD-induced rheological changes of 3T3 ¯broblasts. This next experiment seeks to better understand the e®ect of CytoD on cells. Since there are signi¯cant rheological changes of 3T3 ¯brob- last with CytoD, it is reasonable to assume that this chemical may modify the ¯broblast cytoskeleton. The most important component of the mammalian cell cy- toskeleton is actin, so we will image actin structures in these cells with and without CytoD treatment. The actin cytoskeleton is visualized using phalloidin labeled with Alexa Fluor 532. The excitation maximum is at about 535nm and emission maximum is at about 575nm (see spectra in Fig. 8. Phalloidin is a fungal toxin (small organic molecule) that binds only to poly- merized ¯lamentous actin (F-actin), but not to actin monomers, G-actin. (It is a toxin because it hinders actin disassembly).
image
Figure 8: Excitation and emission spectra for
Alexa Fluor 532.
6.1 Cell ¯xation and labeling protocol
Prepare a dish of ¯xed ¯broblast cells (NIH 3T3) with actin labeled with phalloidin-Alexa Fluor 532. The labeling protocol is as follows: The starting point is as before { cells cultured in dishes containing DMEM++, at approximately 60% con°uency. This is about the optimum percentage of cell population. If cells are too crowded, they will not stretch properly and show their beautiful actin ¯laments. Note also that these cells remain alive until the addition of formaldehyde, therefore requiring that any bu®er/media added to be pre-warmed.
1. Pre-warm the 3.7% formaldehyde solution in a hot water bath on the hotplate. 2. Wash the cells twice with pre-warmed phosphate bu®ered saline (PBS) at pH 7.4. { Remove the medium with a pipette and wash each dish twice with 2 mL of PBS. 3. Fix the samples with 2 mL of 3.7% formaldehyde solution in PBS for 10 minutes at room temperature. { \Fixing means cross-linking the intracellular proteins and freezing the cell structure. This kills the cells. 4. Wash the cells three times with 2 mL PBS. 5. Extract each dish with 2 mL 0.1% Triton X-100 (a type of soap solution in PBS) for 3-5 minutes. { \Extraction refers to partially dissolving the plasma membrane of the cell. 6. Wash the cells 2-3 times with PBS. 7. Incubate the ¯xed cells with 2 mL 1% BSA (bovine serum albumin) in PBS for 20-30 minutes. { BSA blocks the nonspeci¯c binding sites. 8. Wash cells twice with PBS. 9. To each cell dish, add 200 ¹L of °uorescent phalloidin solution (speci¯c binding to F-actin) pre-mixed in methanolic (diluted methanol). Carefully pipet this just onto the central circular glass region of the dish, just enough to cover the cells, and incubate for 60 min. at room temperature. 10. Wash three times with PBS. 11. You can now store the sample at +4±C (normal refrigerator) in PBS for a few days, or in mounting medium for long-term storage (approx. up to 1 year). By a very similar procedure, you should also prepare cells treated with CytoD. Between steps 2 and 3 of staining procedure, add 1 mL of the pre-warmed 10 ¹M CytoD solution for 20 min. Afterwards, wash with DMEM++ twice.
6.2 Actin imaging
Since actin ¯laments and stress ¯bers are nm-scale objects, they are much dimmer than °uorescent beads or the dye solution { care must be taken to get good images of the cytoskeleton. You may need to cover the scope to reduce room light contamination. Adjust the gain and the brightness of the camera to get the best picture. Be sure to keep the same exposure conditions, however, for both untreated and treated cells. Using the 40£ objective, take ¯ve images each of treated and untreated cells. You may have to average multiple captured image frames to obtain acceptable signal to noise levels.
7 Report Requirements
This lab report is due by 12:00 noon (in class) on Thursday, Nov. 30.
7.1 Microscope construction
Make a sketch of your full microscope setup (a hand drawing is perfectly acceptable, but please keep it neat - a ruler is handy) with important parameters indicated (i.e. lens focal lengths, distances of main components, etc.).
7.2 Experiment 1: microscope characterization and Fourier-plane imaging
1. Include your favorite 2-3 white-light images, indicating the magni¯cation and ¯eld of view (do they match what was expected?). 2. Include an uncorrected and a °at-¯eld-corrected °uorescent image of the 4 ¹m beads. 3. Calculate the di®raction-limited resolution for the 10£, 40£ and 100£ objectives, and how this compares with which samples each objective could or could not resolve. 4. Include both the real and Fourier-plane images of the peacock feather and tissue paper, and quantitatively describe how they relate to each other?
7.3 Experiment 2: microrheology measurements by particle tracking
Analyze the bead trajectories for the normal and CytoD-treated cells. Extract their MSD h¢r2i, and the G0 and G00 modulus values, and include enough detail to make it clear how you performed the analysis. Do you get consistent results across multiple cells in each group? What can you tell about the e®ect of CytoD on the mechanical properties of these cells?
7.4 Experiment 3: °uorescence imaging of the actin cytoskeleton
Include one or two of your favorite actin cytoskeleton images from each of the CytoD treated and untreated cell groups. Use your image processing knowledge to optimize image quality. Apply the algorithms you developed during the image processing lab to quantify the degree of \¯berness of the actin structures with and without CytoD. As in Experiment 2, what can you say about the e®ect of CytoD on these cells? Discuss whether the results of this experiment and the microrheology experiment are consistent with each other?
Bonus (optional)
You've now used two di®erent optical microscopy methods to study the e®ects of cytochalasin D on NIH 3T3 ¯broblasts. Hopefully, as you were working, many questions arose in your mind about di®erent cell properties, their underlying physics, experimental conditions, etc. For bonus credit, think about and propose any other experiments you might like to do (using these microscopes, or the AFMs, or any other approach you like) to study any related questions of cytoskeletal mechanics, microrheology, °uorescent labeling, etc. This is not a formal grant proposal { simply outline the question you'd like to answer and suggest an approach/method/technique that you could use to test it.