Difference between revisions of "Assignment 8, Part 2: fabricate a microfluidic device"
Juliesutton (Talk | contribs) (→Overview) |
Juliesutton (Talk | contribs) |
||
| Line 7: | Line 7: | ||
PDMS is a silicone elastomer made by mixing together a viscous liquid base with a crossliking agent. Once mixed and annealed at 60°C, the material will harden into a solid, rubber-like material that is optically clear, non-toxic, and chemically inert. Researchers typically use PDMS for microfluidics because they can use it to cast very small sharp features (down to ~1 micron), and they can covalently bond the PDMS to a glass coverslip, creating a sealed device that is readily compatible with most types of microscopy. | PDMS is a silicone elastomer made by mixing together a viscous liquid base with a crossliking agent. Once mixed and annealed at 60°C, the material will harden into a solid, rubber-like material that is optically clear, non-toxic, and chemically inert. Researchers typically use PDMS for microfluidics because they can use it to cast very small sharp features (down to ~1 micron), and they can covalently bond the PDMS to a glass coverslip, creating a sealed device that is readily compatible with most types of microscopy. | ||
| − | Since our experiment does not require extremely small features, we will simply cut the Y-shaped channel out of a piece of double-sided tape using a laser cutter, rather than casting PDMS over a mold. We're still going to take advantage of PDMS's properties, though. First, it will provide an optically clear structure for our device, and second, because it is somewhat soft and flexible, we can easily punch holes in it (which is hard to do with something like glass) in order to connect our flow channel to inlet and outlet tubing. | + | Since our experiment does not require extremely small features, we will simply cut the Y-shaped channel out of a piece of double-sided tape using a laser cutter, rather than casting PDMS over a mold. We're still going to take advantage of PDMS's properties, though. First, it will provide an optically clear structure for our device, making it compatible for microscopy, and second, because it is somewhat soft and flexible, we can easily punch holes in it (which is hard to do with something like glass) in order to connect our flow channel to inlet and outlet tubing. |
| − | + | ==Cast a slab of PDMS== | |
Two notes before you begin: | Two notes before you begin: | ||
| − | * This protocol has two steps with | + | * This protocol has two steps with 15-60 minute wait times. Part 2 of this assignment can be completed in parallel with Part 1, if you want to keep making progress during the downtime. |
| − | * PDMS is not harmful, but it is viscous and sticky. | + | * PDMS is not harmful, but it is viscous and sticky. Work over a sheet of aluminum foil rather than directly on the work bench, wear gloves when pouring the elastomer base and crosslinking agent, and change them before touching other lab equipment. If you spill any PDMS, clean it up right away with a paper towel or kimwipe. |
Onward! | Onward! | ||
| Line 20: | Line 20: | ||
# Using the scale, measure 31.5 g of the Sylgard™ 184 Silicone Elastomer base into a paper cup. | # Using the scale, measure 31.5 g of the Sylgard™ 184 Silicone Elastomer base into a paper cup. | ||
# Pour in 3.5 g of the curing agent to total 35 g. Note that the curing agent is much less viscous than the base, so pour extra carefully so you don't add too much. | # Pour in 3.5 g of the curing agent to total 35 g. Note that the curing agent is much less viscous than the base, so pour extra carefully so you don't add too much. | ||
| − | # Mix the elastomer base and curing agent REALLY WELL (i.e. | + | # Mix the elastomer base and curing agent REALLY WELL (i.e. for at least 2 minutes) using a plastic stirrer. |
# Pour the mixture into a square petri dish. | # Pour the mixture into a square petri dish. | ||
| − | # Degas the PDMS for | + | # Degas the PDMS for 15 minutes using the vacuum desiccator. Make sure the red T-valve is closed before turning on the vacuum. |
| − | # After | + | # After 15 minutes, turn off the vacuum to the desiccator, and slowly vent the chamber using the red T-valve. |
| − | # Remove | + | # Remove any remaining bubbles with a plastic stirrer. |
| − | # Place the petri dish in the 60°C oven for | + | # Place the petri dish in the 60°C oven for 1 hour. |
The procedure can be stopped at this point and you may store the cured PDMS in the petri dish if you do not have time to complete the next steps in the procedure right away. | The procedure can be stopped at this point and you may store the cured PDMS in the petri dish if you do not have time to complete the next steps in the procedure right away. | ||
| − | + | ==Assemble your device== | |
[[Image: deviceAssemblySchematic.png|thumb|right|400 px|<caption>Microfluidic device assembly.</caption>]] | [[Image: deviceAssemblySchematic.png|thumb|right|400 px|<caption>Microfluidic device assembly.</caption>]] | ||
| Line 35: | Line 35: | ||
# Pick up a rectangle of pre-cut double-sided tape and inspect it to make sure that the channels are clear of excess tape and debris. (Do not yet remove the clear backing of the tape!) | # Pick up a rectangle of pre-cut double-sided tape and inspect it to make sure that the channels are clear of excess tape and debris. (Do not yet remove the clear backing of the tape!) | ||
| − | # Cut out a slab of PDMS | + | # Cut out a slab of PDMS to be the same size as the tape. Leave it in the dish until you are ready to use it, to prevent dust accumulation. |
#* You should be able to cut at least 6 devices out of a single petri dish. You will need to make more of the same devices in future assignments, so plan accordingly. | #* You should be able to cut at least 6 devices out of a single petri dish. You will need to make more of the same devices in future assignments, so plan accordingly. | ||
# Use tweezers to peel off the clear backing from ONE side of the tape. Remove the cut PDMS slab from the petri dish, and stick the flat side (bottom) onto the green tape. Press everywhere to ensure a full seal. | # Use tweezers to peel off the clear backing from ONE side of the tape. Remove the cut PDMS slab from the petri dish, and stick the flat side (bottom) onto the green tape. Press everywhere to ensure a full seal. | ||
| − | # Use a | + | # Use a 1 mm biopsy punch to make holes for the two inlets, and a 0.5 mm biopsy punch to make the outlet hole. |
| + | #* Punch from the bottom side to avoid contaminating the fluidic channels with dust from the table. | ||
| + | #* After inserting the biopsy punch, remove the small core that it creates before extracting the punch from the PDMS. | ||
# Peel off the clear backing from the other side of the tape and seal the flow channel to a 22x40 mm glass coverslip. Apply pressure to the PDMS, rather than the coverslip, to prevent cracking of the coverslip. | # Peel off the clear backing from the other side of the tape and seal the flow channel to a 22x40 mm glass coverslip. Apply pressure to the PDMS, rather than the coverslip, to prevent cracking of the coverslip. | ||
| Line 47: | Line 49: | ||
[[Image: ExampleTubing.jpg|thumb|right|400 px|<caption>Inlet and outlet tubing.</caption>]] | [[Image: ExampleTubing.jpg|thumb|right|400 px|<caption>Inlet and outlet tubing.</caption>]] | ||
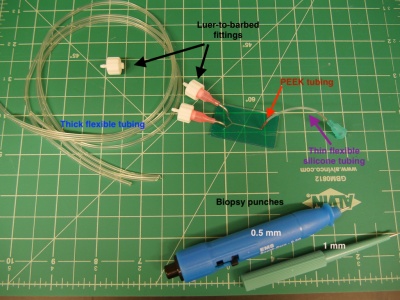
| − | # Using a scalpel, cut the following lengths of | + | # Using a scalpel, cut the following lengths of tubing. Cutting the tubing on an angle will make it easier to sleeve together. |
| − | #* | + | #* 3 x 16" lengths of thick flexible tubing (ID 1/32", OD 3/32"), |
| − | + | #* 1 x 0.5" length of the thin red PEEK tubing (ID 0.005", OD 1/32"), | |
| − | #* | + | #* 1 x 2" length of the thin flexible silicone tubing (ID 0.025", OD 0.047"). |
| − | # | + | # Collect the following: |
| − | + | #* 2 x pink (18G) needles with a 90° bend, | |
| − | # | + | #* 1 x light blue (23G) needle, |
| − | + | #* 3 x luer-to-barbed fittings | |
| − | # | + | # Sleeve the thin flexible silicone tubing over the thin PEEK tubing on one end, and the light blue needle on the other end to make the outlet tubing. Insert the PEEK tubing into the device outlet. |
| + | # Twist the luer-barbed fittings onto the pink needles, then insert the needles into the PDMS inlet holes. | ||
==References== | ==References== | ||
Revision as of 14:59, 12 April 2019
Overview
In this part of the assignment, you will make a microfluidic device out of double-sided tape sandwiched between a glass coverslip and a slab of PDMS. This procedure is incredibly simple, as far as microfluidics go, and was developed in Paul Blainey's lab to image the motion of transport molecules along DNA [1].
PDMS is a silicone elastomer made by mixing together a viscous liquid base with a crossliking agent. Once mixed and annealed at 60°C, the material will harden into a solid, rubber-like material that is optically clear, non-toxic, and chemically inert. Researchers typically use PDMS for microfluidics because they can use it to cast very small sharp features (down to ~1 micron), and they can covalently bond the PDMS to a glass coverslip, creating a sealed device that is readily compatible with most types of microscopy.
Since our experiment does not require extremely small features, we will simply cut the Y-shaped channel out of a piece of double-sided tape using a laser cutter, rather than casting PDMS over a mold. We're still going to take advantage of PDMS's properties, though. First, it will provide an optically clear structure for our device, making it compatible for microscopy, and second, because it is somewhat soft and flexible, we can easily punch holes in it (which is hard to do with something like glass) in order to connect our flow channel to inlet and outlet tubing.
Cast a slab of PDMS
Two notes before you begin:
- This protocol has two steps with 15-60 minute wait times. Part 2 of this assignment can be completed in parallel with Part 1, if you want to keep making progress during the downtime.
- PDMS is not harmful, but it is viscous and sticky. Work over a sheet of aluminum foil rather than directly on the work bench, wear gloves when pouring the elastomer base and crosslinking agent, and change them before touching other lab equipment. If you spill any PDMS, clean it up right away with a paper towel or kimwipe.
Onward!
- Turn on the oven to 60°C (if it isn't on already).
- Using the scale, measure 31.5 g of the Sylgard™ 184 Silicone Elastomer base into a paper cup.
- Pour in 3.5 g of the curing agent to total 35 g. Note that the curing agent is much less viscous than the base, so pour extra carefully so you don't add too much.
- Mix the elastomer base and curing agent REALLY WELL (i.e. for at least 2 minutes) using a plastic stirrer.
- Pour the mixture into a square petri dish.
- Degas the PDMS for 15 minutes using the vacuum desiccator. Make sure the red T-valve is closed before turning on the vacuum.
- After 15 minutes, turn off the vacuum to the desiccator, and slowly vent the chamber using the red T-valve.
- Remove any remaining bubbles with a plastic stirrer.
- Place the petri dish in the 60°C oven for 1 hour.
The procedure can be stopped at this point and you may store the cured PDMS in the petri dish if you do not have time to complete the next steps in the procedure right away.
Assemble your device
- Work on a cutting mat, and not the bench, when punching holes and cutting tubing.
- Pick up a rectangle of pre-cut double-sided tape and inspect it to make sure that the channels are clear of excess tape and debris. (Do not yet remove the clear backing of the tape!)
- Cut out a slab of PDMS to be the same size as the tape. Leave it in the dish until you are ready to use it, to prevent dust accumulation.
- You should be able to cut at least 6 devices out of a single petri dish. You will need to make more of the same devices in future assignments, so plan accordingly.
- Use tweezers to peel off the clear backing from ONE side of the tape. Remove the cut PDMS slab from the petri dish, and stick the flat side (bottom) onto the green tape. Press everywhere to ensure a full seal.
- Use a 1 mm biopsy punch to make holes for the two inlets, and a 0.5 mm biopsy punch to make the outlet hole.
- Punch from the bottom side to avoid contaminating the fluidic channels with dust from the table.
- After inserting the biopsy punch, remove the small core that it creates before extracting the punch from the PDMS.
- Peel off the clear backing from the other side of the tape and seal the flow channel to a 22x40 mm glass coverslip. Apply pressure to the PDMS, rather than the coverslip, to prevent cracking of the coverslip.
Your device is now assembled!
Connect tubing
- Using a scalpel, cut the following lengths of tubing. Cutting the tubing on an angle will make it easier to sleeve together.
- 3 x 16" lengths of thick flexible tubing (ID 1/32", OD 3/32"),
- 1 x 0.5" length of the thin red PEEK tubing (ID 0.005", OD 1/32"),
- 1 x 2" length of the thin flexible silicone tubing (ID 0.025", OD 0.047").
- Collect the following:
- 2 x pink (18G) needles with a 90° bend,
- 1 x light blue (23G) needle,
- 3 x luer-to-barbed fittings
- Sleeve the thin flexible silicone tubing over the thin PEEK tubing on one end, and the light blue needle on the other end to make the outlet tubing. Insert the PEEK tubing into the device outlet.
- Twist the luer-barbed fittings onto the pink needles, then insert the needles into the PDMS inlet holes.
References
- ↑ | K. Xiong and P. C. Blainey, “A Simple, Robust, and High Throughput Single Molecule Flow Stretching Assay Implementation for Studying Transport of Molecules Along DNA,” J. Vis. Exp., no. 128, pp. 1–7, 2017
- Overview
- Part 1: feedback systems
- Part 2: fabricate a microfluidic device
- Part 3: add flow control and test your device
Back to 20.309 Main Page