20.109(S22):M2D5
Contents
Introduction
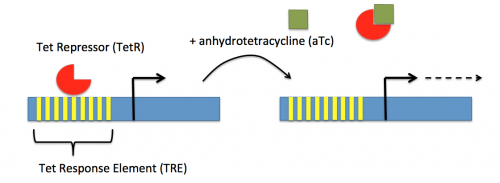
In contrast, expression of the gene encoding dCas9 within pdCas9 is regulated by an inducible promoter (pLtetO-1). An inducible promoter is 'off' unless the appropriate molecule is present to relieve repression. In the case of the CRISPRi system, expression of the gene encoding dCas9 is inhibited due to the use of a tet-based promoter construct. Tet is shorthand for tetracycline, which is an antibiotic that inhibits protein synthesis through preventing the association between charged aminoacyl-tRNA molecules and the A site of ribosomes. Bacterial cells that carry the tet resistance cassette are able to survive exposure to tetracycline by expressing genes that encode an efflux pump that 'flushes' the antibiotic from the bacterial cell. To conserve energy, the tet system is only expressed in the presence of tetracyline. In the absence of tetracycline, a transcription repressor protein (TetR) is bound to the promoter upstream of the tet resistance cassette genes. When tetracycline is present, the molecule binds to TetR causing a confirmational change that results in TetR 'falling off' of the promoter. In the CRISPRi system, the tet-based promoter construct upstream of the gene that encodes dCas9 is 'off' unless anhydrotetracyline (aTc), an analog of tetracyline, is added to the culture media. Why is it important to use an analog rather than the actual antibiotic?Taken together, the sgRNA_target is constitutively transcribed and, thereby, always present. The dCas9 protein is only present when aTc is added. Thus, gene expression is only altered when aTc is present. In this, when dCas9 is expressed it forms a complex with the sgRNA_target. The sgRNA target then 'seeks out' the target within the host genome. When the targeted sequence is recognized, the complex binds and acts as a 'roadblock' by prohibiting RNAP access to the sequence. Because the targeted gene is not able to be transcribed, the protein encoded by that gene is not synthesized. In our experiments, we hypothesize that the absence of specific proteins, or enzymes, involved in anaerobic fermentative metabolism will increase the yield of ethanol.
Protocols
Prepare media for ethanol yield experiment
To test the effect of sgRNA_target on increasing the production of ethanol, the co-transformed E. coli MG1655 cells both with and without oxygen. Remember from lecture that cells grown anaerobically should produce more fermentation products; however, the goal of using the CRISPRi system is to further enhance the production of ethanol by manipulating gene expression of an enzyme in the anaerobic fermentative pathway.
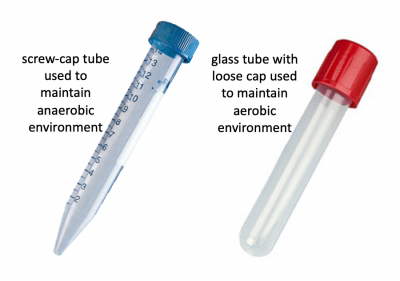
Before we look at the specific conditions that were tested, let's first review how anaerobic and aerobic cultures were maintained. Rather than using anaerobic chambers or gas replacement, a much simpler technique was employed to omit oxygen from the cultures for this experiment. Because screw-cap tubes maintain a tight seal, cultures can be maintained in a low-O2 environment. For this technique cultures are grown at least 24 hrs to ensure the following: 1. that the oxygen present at the time of inoculation is depleted by the growing cells and 2. that the cells grow in the O2-depleted environment long enough to undergo anaerobic fermentative metabolism. Aerobic cultures were maintained using standard glass test tubes with loose-fitting caps. Both culture tubes are shown in the image to the right.In total, eight conditions were tested for each sgRNA_target:
- MG1655 +O2 -aTc (glass tube)
- MG1655 -O2 -aTc (screw-cap tube)
- MG1655 +O2 +aTc (glass tube)
- MG1655 -O2 +aTc (screw-cap tube)
- MG1655 +CRISPRi +O2 -aTc (glass tube)
- MG1655 +CRISPRi -O2 -aTc (screw-cap tube)
- MG1655 +CRISPRi +O2 +aTc (glass tube)
- MG1655 +CRISPRi -O2 +aTc (screw-cap tube)
All cultures were prepared in 5 mL LB broth containing 25 μg/mL of chloramphenicol and 100 μg/mL of ampicillin.
In your laboratory notebook, complete the following:
- For each of the conditions that were tested, answer the following questions:
- Is the cell culture using anaerobic fermentative metabolism?
- Do the cells contain the CRISPRi system?
- Is the CRISPRi system active (are the components expressed)?
- Which culture conditions provide controls? What does each control indicate / validate?
- In which culture condition do you expect to see the lowest yield of ethanol? Why?
- In which culture condition do you expect to see the highest yield of ethanol? Why?
Part 4: Align sgRNA_target sequences to host genome
Next, you will use your knowledge of primer design to align the sgRNA_target sequences that were designed by former 109ers to the targeted genes in the MG1655 genome. Recall that your goal in this module is to optimize the CRISPRi system by building on the data collected by students in previous semesters. The first step in achieving this goal is mining the data that exist! The sgRNA_target sequences that you will assess are included in the table below:
With your laboratory partner, align the sgRNA_target sequences with the targeted genes. Feel free to divide the workload, one partner can align the sequences that target ldhA and the other can align the sequences that target pta-ack.
- Use the KEGG Database to obtain the DNA sequences of the targeted genes (ldhA and pta-ack) in the E. coli K-12 MG1655 strain.
- Enter the name of targeted gene in the Search genes box and click Go.
- Double click on the linked gene name.
- In your laboratory notebook, use the information provided in the KEGG database to answer the following questions:
- What is the full name of the gene (or Definition)?
- In what pathways is the gene involved?
- The amino acid (AA) sequence and nucleotide sequence (NT) for the gene are provided at the bottom of the page.
- Generate a new DNA file in SnapGene that contains the NT sequence of the gene.
- Because sgRNA_target molecules were generated that target the promoter, enter 50 in the +upstream box to get the 50 basepair sequence immediately preceding the start codon.
- Identify the sgRNA_target sequences from the table below in the MG1655 targeted gene.
- For each sgRNA_target sequence, create a feature in the SnapGene file.
- In your laboratory notebook, complete the following:
- Attach the SnapGene file with the sgRNA_target sequences aligned.
- Draw a simplified schematic that shows sgRNA_target sequence alignments. For an example, refer back to Fig. 2C and 2D from the Lei et. al. article.
- Determine which sgRNA_target sequences bind downstream of a PAM sequence and indicate this information on the schematic.
- Speculate on which sgRNA_target sequences might be better at increasing ethanol yield. Include reasons for why!
Reagents list
- chloramphenicol antibiotic (from Sigma)
- ampicillin antibiotic (from Sigma)
- anhydrotetracycline (aTc) (from Sigma)
Next day: Complete CRISPRi experiment and measure fermentation products