20.109(S22):M2D5
Contents
Introduction
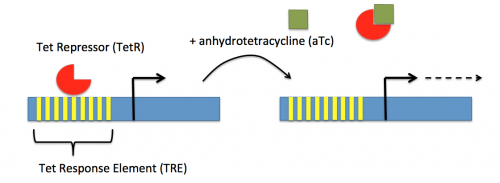
In contrast, expression of the gene encoding dCas9 within pdCas9 is regulated by an inducible promoter (pLtetO-1). An inducible promoter is 'off' unless the appropriate molecule is present to relieve repression. In the case of the CRISPRi system, expression of the gene encoding dCas9 is inhibited due to the use of a tet-based promoter construct. Tet is shorthand for tetracycline, which is an antibiotic that inhibits protein synthesis through preventing the association between charged aminoacyl-tRNA molecules and the A site of ribosomes. Bacterial cells that carry the tet resistance cassette are able to survive exposure to tetracycline by expressing genes that encode an efflux pump that 'flushes' the antibiotic from the bacterial cell. To conserve energy, the tet system is only expressed in the presence of tetracyline. In the absence of tetracycline, a transcription repressor protein (TetR) is bound to the promoter upstream of the tet resistance cassette genes. When tetracycline is present, the molecule binds to TetR causing a confirmational change that results in TetR 'falling off' of the promoter. In the CRISPRi system, the tet-based promoter construct upstream of the gene that encodes dCas9 is 'off' unless anhydrotetracyline (aTc), an analog of tetracyline, is added to the culture media. Why is it important to use an analog rather than the actual antibiotic?Taken together, the sgRNA_target is constitutively transcribed and, thereby, always present. The dCas9 protein is only present when aTc is added. Thus, gene expression is only altered when aTc is present. In this, when dCas9 is expressed it forms a complex with the sgRNA_target. The sgRNA target then 'seeks out' the target within the host genome. When the targeted sequence is recognized, the complex binds and acts as a 'roadblock' by prohibiting RNAP access to the sequence. Because the targeted gene is not able to be transcribed, the protein encoded by that gene is not synthesized. In our experiments, we hypothesize that the absence of specific proteins, or enzymes, involved in anaerobic fermentative metabolism will increase the yield of ethanol.
Protocols
Part 4: Align sgRNA_target sequences to host genome
To ensure that you understand the principles of using sgRNA to target a gene of interest, complete the sgRNA Design Worksheet with your laboratory partner (linked here). It may be helpful to review the work you completed in Part 2 of the previous laboratory session.
In your laboratory notebook, attach the completed sgRNA Design Worksheet.
Next, you will use your knowledge of primer design to align the sgRNA_target sequences that were designed by former 109ers to the targeted genes in the MG1655 genome. Recall that your goal in this module is to optimize the CRISPRi system by building on the data collected by students in previous semesters. The first step in achieving this goal is mining the data that exist! The sgRNA_target sequences that you will assess are included in the table below:
With your laboratory partner, align the sgRNA_target sequences with the targeted genes. Feel free to divide the workload, one partner can align the sequences that target ldhA and the other can align the sequences that target pta-ack.
- Use the KEGG Database to obtain the DNA sequences of the targeted genes (ldhA and pta-ack) in the E. coli K-12 MG1655 strain.
- Enter the name of targeted gene in the Search genes box and click Go.
- Double click on the linked gene name.
- In your laboratory notebook, use the information provided in the KEGG database to answer the following questions:
- What is the full name of the gene (or Definition)?
- In what pathways is the gene involved?
- The amino acid (AA) sequence and nucleotide sequence (NT) for the gene are provided at the bottom of the page.
- Generate a new DNA file in SnapGene that contains the NT sequence of the gene.
- Because sgRNA_target molecules were generated that target the promoter, enter 50 in the +upstream box to get the 50 basepair sequence immediately preceding the start codon.
- Identify the sgRNA_target sequences from the table below in the MG1655 targeted gene.
- For each sgRNA_target sequence, create a feature in the SnapGene file.
- In your laboratory notebook, complete the following:
- Attach the SnapGene file with the sgRNA_target sequences aligned.
- Draw a simplified schematic that shows sgRNA_target sequence alignments. For an example, refer back to Fig. 2C and 2D from the Lei et. al. article.
- Determine which sgRNA_target sequences bind downstream of a PAM sequence and indicate this information on the schematic.
- Speculate on which sgRNA_target sequences might be better at increasing ethanol yield. Include reasons for why!
Part 2: Examine pgRNA sequencing results
Your goal today is to analyze the sequencing data for you two potential mutant pgRNA clones - two independent colonies from your amplification reaction - and then decide which colony to proceed with for the CRISPRi manipulation of the E. coli MG1655 fermentation pathway.
Retrieve sequence results from Genewiz
- Your sequencing data is available from Genewiz. For easier access, the information was uploaded to the Class Data Page.
- Download the zip folder with your team sequencing results and confirm that there are 8 files saved in the folder.
- For each sequencing reaction, you should have one .abi file and one .seq file.
- Open one of the .abi files.
- This file contains the chromatogram for your sequencing reaction. Scroll through the sequence and ensure that the peaks are clearly defined and evenly spaced. Low signal (or peaks) or stacked peaks can provide incorrect base assignments in the sequence.
- Open one of the .seq files.
- This file contains the base assignments for your sequencing reaction. The bases are assigned by the software from the chromatogram sequence.
- The start of the a sequencing reaction result often contains several Ns, which indicates that the software was unable to assign a basepair. Given the chromatogram result, why might the software assign Ns in this region of the sequence?
- Include all of your observations in your Benchling notebook. You can also attach the files to your entry.
Confirm gRNA sequence in pgRNA using SnapGene
You should align your sequencing data with a known sequence, in this case the gRNA target sequence you selected, to identify any unintended base changes that may have occurred. There are several web-based programs for aligning sequences and still more programs that can be purchased. The steps for using SnapGene are below. Please feel free to use any program with which you are familiar.
- Generate a new DNA file that contains the gRNA oligo you designed on M2D3. This file should contain only the target sequence you selected and the dCas9 tag sequence (not the plasmid sequence).
- Generate an additional new DNA file that contains the results from the sequencing reaction completed by Genewiz.
- For each sequencing result you should generate a distinct new DNA file. Remember you should have a forward and reverse sequencing result for each of your clones!
- Paste the sequence text from your sequencing run into the new DNA file window. If there were ambiguous areas of your sequencing results, these will be listed as "N" rather than "A" "T" "G" or "C" and it's fine to include Ns in the query.
- The start and end of your sequencing may have several Ns. In this case it is best to omit these Ns by pasting only the 'good' sequence that is flanked by the ambiguous sequence.
- To confirm the gRNA sequence in your clones, open one of the forward sequencing results files generated in the previous step.
- Select 'Tools' --> 'Align to Reference DNA Sequence...' --> 'Align Full Sequences...' from the toolbar.
- In the window, select the file that contains the gRNA oligo sequence and click 'Open'.
- A new window will open with the alignment of the two sequences. The top line of sequence shows the results of the sequencing reaction and the bottom line shows the oligo you designed.
- Are there any discrepancies or differences between the two sequences? Scroll through the entire alignment to check the full sequencing result and note any basepair changes.
- Follow the above steps to examine all of your sequencing results. Remember: you used a forward and a reverse primer to interrogate both potential gRNA_target plasmids.
- You should save a screenshot of each alignment and attach them to your Benchling notebook.
If both clones for your gRNA_target have the correct sequence, choose either co-transformant to use for the aTc induction step. If only one is correct, then this is the co-transformant you will use next time. If neither of your plasmids carry the appropriate insertion, talk to the teaching faculty.
Reagents list
- chloramphenicol antibiotic (from Sigma)
- ampicillin antibiotic (from Sigma)
- anhydrotetracycline (aTc) (from Sigma)
Next day: Complete CRISPRi experiment and measure fermentation products