20.109(S21):M3D3
Contents
Introduction
Now that you have prepared DNA encoding your mutant inverse pericams, we need to produce the proteins so we can assess the affinity and cooperativity of your new protein. Since the last time you were here, your oh-so devoted teaching staff transformed competent bacteria (strain NEB 5α) with your SDM product. Successfully transformed NEB 5α bacteria grew into colonies on ampicillin-containing plates, and two colonies were picked to grow in liquid cultures. The liquid culture of E. coli serves as a plasmid-generating factory. Today you will purify the plasmids carrying the hopefully mutated inverse pericam gene using a Qiagen mini-prep kit. To ensure that the SDM reaction was successful, and therefore the IPC gene was mutated, you will prepare the purified DNA for sequencing analysis. Though the NEB 5α cell strain is able to replicate the inverse pericam plasmid DNA, it cannot produce the inverse pericam protein. Therefore, you will transform your IPC mutant plasmids into a new bacterial system, BL21(DE3)pLysS, that can produce the protein for later analysis.
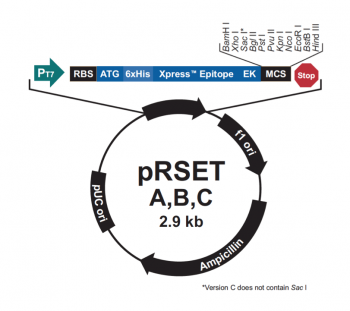
The bacterial expression vector we are using, pRSET, encodes many features that make it ideal for our research purposes. To enable selection of bacterial cells that carry the plasmid, an antibiotic cassette (specifically, an ampicillin marker) is included on the vector. Several features are included to promote protein expression and purification. In the image to the right a schematic representation of these features is shown. The T7 promoter drives expression of the gene that encodes IPC (or your mutated IPC). To ensure that the transcript is translated into a protein, a Ribosome Binding Site (RBS) is included. The ATG sequence serves as the transcriptional start and the 6xHis represents the six-histidine residue tag that is used for protein purification via affinity chromatography.
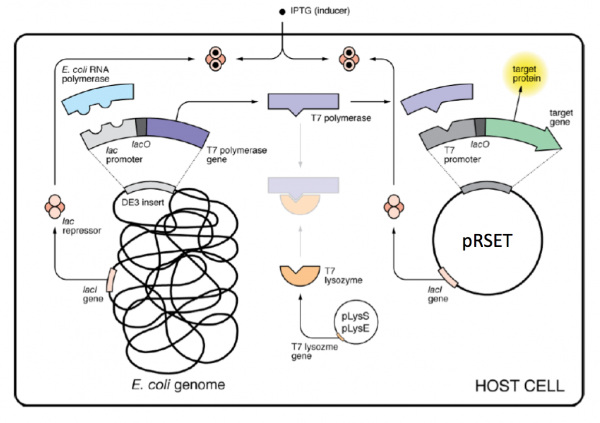
As mentioned above, pRSET encodes the bacteriophage T7 promoter, which is active only in the presence of T7 RNA polymerase (T7RNAP), an enzyme that therefore must be expressed by the bacterial strain used to make the protein of interest. We will use the BL21(DE3)pLysS strain, which has the following genotype: F-, ompT hsdSB (rB- mB-) gal dcm (DE3) pLysS (CamR). In BL21(DE3), T7RNAP is associated with a lac construct. Constitutively expressed lac repressor (lacI gene) blocks expression from the lac promoter; thus, the polymerase will not be produced except in the presence of repressor-binding lactose or a small-molecule lactose analogue such as IPTG (isopropyl β-D-thiogalactoside). To reduce ‘leaky’ expression of the protein of interest (in our case, inverse pericam), the pLysS version of BL21(DE3) contains T7 lysozyme, which inhibits basal transcription of T7RNAP. This gene is retained by chloramphenicol selection, while the pRSET plasmid itself (and thus inverse pericam) is retained by ampicillin selection.
After completing mini-preps to isolate your plasmid DNA today (two mutant pRSET-IPC candidates), you will prepare the DNA for sequencing analysis, as well as use it immediately for transformation. In order to transform BL21(DE3)pLysS cells with your mutant IPC plasmids, you will first have to make the cells competent, i.e., able to efficiently take up foreign DNA. With the NEB 5α strain, we used commercially available competent cells that did not need further treatment prior to DNA addition. Today, you will make chemically competent cells yourself using calcium chloride, then incubate them with plasmid DNA and heat shock them as before prior to plating. Whether prepared by a company or by you, remember that competent cells are extremely fragile and should be handled gently, i.e. kept cold and not vortexed. Bacterial transformation is efficient enough for most lab purposes, resulting in as many as 109 transformed cells per microgram of DNA, but even with highly competent cells only 1 DNA molecule in about 10,000 is successfully transformed.
After today's lab session, the teaching staff will pick colonies and set up liquid overnight cultures from your transformed BL21(DE3)pLysS cells. Next time, you will add IPTG to these liquid cultures to induce expression of your mutant proteins, which you will then isolate and characterize.
Protocols
Part 1: Participate in Comm Lab workshop
Our communication instructors, Dr. Prerna Bhargava and Dr. Sean Clarke, will join us today for a discussion on preparing a Research proposal presentation.
Part 2: Induce expression of IPC variants
Part 3: Purify IPC variants
Reagents list
Next day: Evaluate effect of mutations on IPC variants