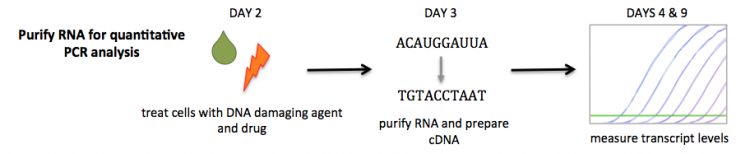
20.109(S18):Induce DNA damage and apply drug treatments for RNA purification (Day2)
Introduction
Protocols
Part 1: Induce DNA damage
To begin your assay, you will first induce DNA damage by incubating your cells with etoposide. Etoposide is itself a chemotherapy drug that generates DNA stand breaks by forming a ternary complex with DNA and topoisomerase II. This prevents religation of the DNA strands after unwinding. This effects cancer cells more than non-cancerous cells because cancer cells divide much faster. We will use etoposide to damage the DNA and then treat with an additional NHEJ-targeting drug, either mibefradil or loperamide. The mechanism by which mibefradil and loperamide target NHEJ is unknown (and therefore one of our research questions), though both drugs are useful in the treatment of other conditions:
- Mibefradil is is a calcium channel blocker used to treat hypertension and chronic angina pectoris.
- Loperamide is known to slow the contractions of the intestines and is used to treat gastroenteritis, inflammatory bowel disease, and short bowel syndrome.
Before you begin, select which drug (mibefradil or loperamide) you will use for your experiments in this module and enter your team information in the table below.
T/R section
| Mibefradil | Loperamide | |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 |
W/F section
| Mibefradil | Loperamide | |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 |
To measure gene expression of a DNA cell cycle checkpoint factor, you will perform quantitative PCR (qPCR). In qPCR, the amount of a specific transcript can be measured. The presence of checkpoint factors in an indication of DNA damage in that the cell cycle is halted when excessive damage is incurred by a cell. For your experiments, the results of p21 expression levels will serve as a control that DNA damage was indeed induced following etoposide and etoposide + drug treatment. You will also complete qPCR analysis to assess the expression levels of a gene you select in the subsequent laboratory sessions.
In this exercise, you will induce DNA damage in cells that were seeded by the teaching faculty then add the drug that you selected above. Next class you will purify RNA from your cells for quantitative PCR analysis.
- Prepare your working space within the tissue culture hood.
- Calculate the volume of etoposide stock needed for DNA damage induction.
- Obtain an aliquot of pre-warmed media from the 37 °C water bath (9 mL).
- Determine the volume of etoposide stock (100 mM) you need to add to the media for a final concentration of 100 μM.
- Retrieve four T75 flasks (two DLD-1 cultures and two BRCA2- cultures) from the 37 °C incubator and visually inspect your cells with a microscope.
- Record your observations concerning media color, confluency, etc. in your laboratory notebook.
- Move your flasks into the tissue culture hood.
- Aspirate the spent media from each flask.
- Be careful not to cross-contaminant between flasks with different cell lines!
- Add 5 mL of PBS to each flask and rock the plate gently to wash the cells.
- Aspirate the PBS from each flask.
- Again, be careful not to cross-contaminate.
- Add 5 mL of the media containing etoposide that you prepared in Step #2.
- Carefully put your flasks in the 37 °C incubator for 60 min.
- Assist you partner if they are still working. When you are both done, return to the main laboratory space.
- Return to the tissue culture space and retrieve your flasks from the 37 °C incubator.
- Aspirate the media containing etoposide from each flask.
- Be mindful of cross-contamination.
- Add 10 mL of fresh media to each flask.
- Label one DLD-1 and one BRCA2- flask '+etop' to denote that the cells were treated with only etoposide. Move these flasks to the 37 °C incubator.
- These cultures are the 'no drug treatment' controls.
- Label the remaining two flasks '+etop, +mib' or '+etop, +lop' depending on which drug you selected.
- Calculate the volume of drug you need to add to your flasks for a final mibefradil concentration of 4.1 μM or loperamide concentration of 2.8 μM.
- Be sure to use the concentrations appropriate for the drug you chose.
- The stock concentrations of mibfradil and loperamide are 10 mM.
- It may be helpful to dilute the drug stocks to avoid pipetting very small volumes.
- Add the appropriate volume of drug and move your flasks to the 37 °C incubator.
- Assist your laboratory partner, if necessary, then clean the hood and return the main laboratory space.