Difference between revisions of "20.109(S09):Module 1"
| Line 5: | Line 5: | ||
==M o d u l e 1== | ==M o d u l e 1== | ||
| − | + | '''Instructors:''' [http://web.mit.edu/be/people/jasanoff.htm Alan Jasanoff] and [[User:AgiStachowiak| Agi Stachowiak]] | |
| − | + | ||
| − | + | '''TA:''' [[Naiyan Chen]] | |
| − | + | ||
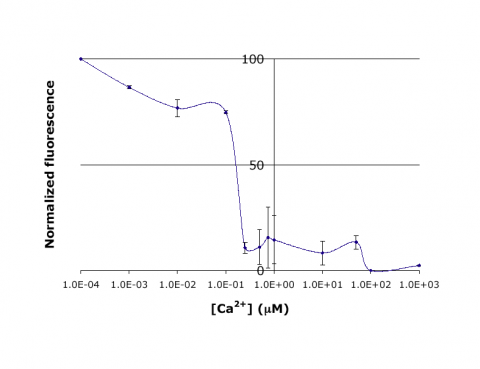
| − | + | In this experiment, you will modify a protein called inverse pericam (developed by[http://www.ncbi.nlm.nih.gov/pubmed/11248055 Nagai et al.]) in order to affect its functions as a sensor. Inverse pericam (IPC) comprises a permuted fluorescent protein linked to a calcium sensor. The “inverse” in the name refers to the fact that this protein shines brightly in the absence of calcium, but dimly once calcium is added. The dissociation constant <math>K_D</math> of wild-type IPC with respect to calcium is reported to be 0.2 μM (see also figure below). Your goal will be to shift this titration curve or change its steepness by altering one of the calcium binding sites in IPC’s calcium sensor portion. You will modify inverse pericam at the gene level using a process called site-directed mutagenesis, express the resultant protein in a bacterial host, and finally purify your mutant protein and assay its calcium-binding activity via fluorescence. In the course of this module, we will consider the benefits and drawbacks of different approaches to protein design, and the types of scientific investigations and applications enabled by fluorescently tagged biological molecules. | |
| + | |||
| + | We gratefully acknowledge 20.109 instructor Natalie Kuldell for helpful discussions during the development of this module, as well as for her prior work in developing a [http://openwetware.org/wiki/20.109:Module_2 related module]. | ||
| + | |||
| + | [[Image:20.109 Ca-IPC-Titr-Fig.png|thumb|center|480px|'''Titration curve for IPC.''' Shown here is sample data from the teaching lab: normalized fluorescence for wild-type inverse pericam as a function of calcium concentration. As you will later learn, an apparent <math>K_D</math> can be estimated from such a plot: it is the point on the ''x''-axis where the curve crosses ''y'' = 50%, or ~0.1 μM here.]] | ||
| + | |||
| + | [[20.109(S09):Start-up protein engineering (Day1)| Module 1 Day 1: Start-up protein engineering]]<br> | ||
Revision as of 17:51, 30 September 2008
M o d u l e 1
Instructors: Alan Jasanoff and Agi Stachowiak
TA: Naiyan Chen
In this experiment, you will modify a protein called inverse pericam (developed byNagai et al.) in order to affect its functions as a sensor. Inverse pericam (IPC) comprises a permuted fluorescent protein linked to a calcium sensor. The “inverse” in the name refers to the fact that this protein shines brightly in the absence of calcium, but dimly once calcium is added. The dissociation constant $ K_D $ of wild-type IPC with respect to calcium is reported to be 0.2 μM (see also figure below). Your goal will be to shift this titration curve or change its steepness by altering one of the calcium binding sites in IPC’s calcium sensor portion. You will modify inverse pericam at the gene level using a process called site-directed mutagenesis, express the resultant protein in a bacterial host, and finally purify your mutant protein and assay its calcium-binding activity via fluorescence. In the course of this module, we will consider the benefits and drawbacks of different approaches to protein design, and the types of scientific investigations and applications enabled by fluorescently tagged biological molecules.
We gratefully acknowledge 20.109 instructor Natalie Kuldell for helpful discussions during the development of this module, as well as for her prior work in developing a related module.