Difference between revisions of "20.109(F23):M2D7"
Noreen Lyell (Talk | contribs) (Created page with "<div style="padding: 10px; width: 820px; border: 5px solid #434a43;"> {{Template:20.109(F23)}} ==Introduction== The research goal for this module was to identify small mo...") |
Becky Meyer (Talk | contribs) (→Part 1: Consider modifications that could improve binding to PfFKBP35) |
||
| (15 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
==Introduction== | ==Introduction== | ||
| − | The | + | Research takes time! The work that is reported in a published article is often a culmination of years of study by multiple investigators. Because of this it is very difficult to complete an entire project in the time span of a single semester. The work you completed this semester is part of an ongoing research project that started even before your previous classmates. |
| − | + | '''Previous work''' | |
| + | In work completed by the Fall 2022 class, students screened a library of small molecules that are chemically altered versions of FK506 which is known to bind to PfFKBP35 and the human ortholog, FKBP12. The goal of the initial screen was to examine the binding of each small molecule to PfFKBP35 and to FKBP12 in an effort to identify molecules that could potentially be used to target PfFKBP35 preferentially over FKBP12. From this experiment, the four small molecules used in your research were identified. | ||
| − | + | '''Your work''' | |
| + | The research goal for this module was to further study small molecules that were previously identified to bind to PfFKBP35. To better characterize the binding parameters of each of the four identified small molecules to PfFKBP35, you examined binding across different concentrations of small molecule. The T<sub>m</sub> for PfFKBP35 across a range of small molecule concentrations can be used to confirm that binding is occurring and can also give some insight into the strength of the binding. If the small molecule binds to FfFKBP35, then a shift in the T<sub>m</sub> will be observed and can be quantified by subtracting the T<sub>m</sub> of PfFKBP35 without small molecule. The strength of binding can qualitatively be addressed by considering the concentrations of small molecule needed to cause a shift in the T<sub>m</sub>. If a shift in T<sub>m</sub> for a small molecule is observed at only high concentrations, then perhaps the strength of binding is not as good as for another small molecule that causes a shift in T<sub>m</sub> at lower concentrations. | ||
| − | + | '''Future work''' | |
| + | There are several next steps that can be taken from your work. An obvious experiment is to test the small molecules used in your work with FKBP12 to confirm that the binding to PfFKBP35 is preferential across a range of concentrations of small molecule. If a small molecule passes this step, then perhaps modifications can be made to further improve the affinity and specificity to PfFKBP35. If a small molecule doesn't emerge as promising, then perhaps it is time to restart the screening process to identify new molecules. | ||
==Protocols== | ==Protocols== | ||
| − | ===Part 1: | + | ===Part 1: Consider modifications that could improve binding to PfFKBP35=== |
| − | + | One method used to identify small molecules that bind to a specific target is to rationally design small molecules based on the structure information. If the structure of the protein target is known, then this information can be used to build a molecule that binds. In reality this is a very challenging task! To increase the chances of success using rational design, a scaffold molecule that is a known binder can be used. This is the approach that was used to develop the initial library of molecules that was screened by students in Fall 2022. The library was built by chemically modifying FK506, which is known to bind to PfFKBP35. This approach is only useful when a known small molecule binder is known. In addition, using this approach is not be useful in discovering molecules that bind outside of a known binding pocket or active site. | |
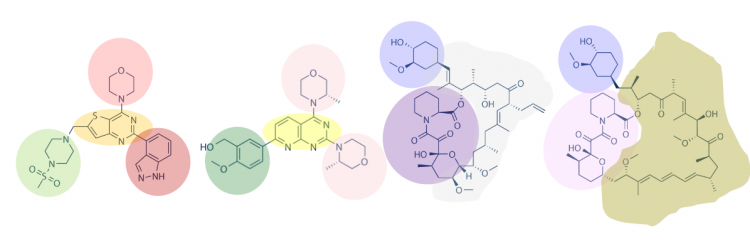
| − | + | One method for assessing what functional groups are potentially important in protein-small molecule binding is to visually inspect known small molecule binders for common features / structures. As shown in the image below, common shapes can be identified between the small molecules. | |
| − | + | [[Image:Sp17 20.109 M1D7 chemical structure features.png|thumb|750px|center|]] | |
| − | + | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | *For the small molecules that showed binding from the class DSF results, | |
| + | **Are there any common features present? | ||
| + | **If there are common features, do these features resemble the functional groups you hypothesized would promote binding to PfFKBP35 in the exercise completed on M2D4? | ||
| + | **What might this suggest about the potential binding site(s) on PfFKBP35? | ||
| + | *How might you make modifications to the small molecules / features to improve binding to PfFKBP35? | ||
| + | *What is a downside of using this approach to identify small molecules that bind a specific target? | ||
| + | *What is an upside of using this approach to identify small molecules that bind a specific target? | ||
| − | + | ===Part 2: Consider other methods for small molecule discovery=== | |
| − | + | In contrast to rational design, another method used to find small molecules that bind a protein of interest is to use a high-throughput screen, such as the small molecular microarray (SMM). Though we did not use this method as part of this module, the technology and the data analysis workflow will be briefly described below as this could be an alternative strategy to the approach used in this module. This technology enables researchers to screen thousands, to hundreds of thousands, of small molecules in an unbiased manner using commercially available libraries. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | '''Preparing the small molecule microarray''' <br> | |
| + | An SMM screen requires the covalent attachment of a library of small molecules to a glass slide. The library is meant to broadly sample the drug-like chemical space (''i.e.'' all possible chemical structures that have drug-like physical properties) and contains about 50,000 small molecules. Some libraries are much smaller, while many pharmaceutical companies possess high-throughput screening (HTS) collections of millions of compounds. Because this chemical space is very large, it’s difficult to generalize any single chemical reaction for this attachment that can be applied to all small molecules. This method uses a “one-size-fits-most” approach, where the glass slide is functionalized with a broadly reactive electrophile capable of reacting with nucleophiles present in most drug-like small molecules, such as alcohols or amines. Many small molecules contain multiple nucleophiles suitable for attachment. In this case, manufacturing will result in a mixture of attachment sites. It’s important to remember that attachment to the glass slide constrains the possible orientation of the protein-small molecule interaction; some orientations are not possible because the glass slide and linker are in the way. | ||
| − | + | First, a glass slide with exposed amines across the surface and attach a short PEG (polyethylene glycol) linker. To the end of this PEG linker, an isocyanate group is attached. Isocyanate, or R-N=C=O, is a resonant structure, and a partial positive charge is stabilized on the carbon atom. This carbon atom is electrophilic, and small molecules with nucleophiles will react here. It is estimated that about 70% of drug-like small molecules are amenable to this reaction, and the library is filtered to contain only these molecules. | |
| − | + | [[Image:Sp17 20.109 M1D4 SMM printing.png|thumb|700px|center|Image from Bradner, J. E. et al. [http://www.ncbi.nlm.nih.gov/pubmed/17406478 PMID: 17406478]]] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | ===Part | + | The compound library is dissolved in DMSO and stored in 384-well plates. To dispense the compounds onto our functionalized glass slide, a robotic arm with a set of 48 metal pins is used to transfer the compounds to the glass slide. Each metal pin has a small slit in the end, and capillary action is used to precisely withdraw and dispense consistent volumes. When the pins touch the glass slide, the compound in DMSO is dispensed into a small circle of approximately 160 micron in diameter. Each pin prints one compound in two different locations on each slide, and then the pins are washed in water and DMSO. This process is repeated for each compound, resulting in our final microarray. The microarray is divided into 48 subarrays, and each subarray corresponds to one pin and contains 256 discrete spots. Within each subarray, we print a set of fluorescent compounds in the shape of an ‘X’ so that we can precisely determine where each spot is printed. After the compounds react, we quench the surface so that no electrophiles remain. This results in our final microarray; a collection of approximately 12,000 discrete spots displaying one compound each. |
| + | |||
| + | '''Performing an SMM screen''' <br> | ||
| + | |||
| + | <font color = #0d368e>'''To see the steps used to screen small molecules, please watch the video tutorial linked here: [[https://www.dropbox.com/s/0phy1rg1w23fs49/SMM%20Staining.mp4?dl=0 SMM Screen]]. The steps are detailed below so you can follow along!'''</font color> | ||
| + | |||
| + | '''Scanning SMM slides''' <br> | ||
| + | |||
| + | After the printed SMM slides are incubated with purified protein, the next step is to check which of the small molecules, if any, you screened may be able to bind the protein of interest. To do this the slides are imaged using a Genepix microarray scanner. The scanner measures the fluorescence signal emitted from the slide at two wavelengths: 532 nm | ||
| + | and 635 nm. The goal for today is to familiarize you with how the SMM slides are scanned and imaged. These images are the raw data that will be used to identify putative small molecule binders. | ||
| + | |||
| + | [[Image:Fa22 M2D4 SMM, scan.png|thumb|700px|center|'''Overview of SMM slide imaging.''' A. To visualize which small molecule is putative binder of PfFKBP35, the 6xHis-tag is labeled using an anti-His antibody that is conjugated to a fluorophore. B. The fluorophore used to label protein bound to the small molecule in a particular location on the slide emits a red signal (indicated by white arrow). Image in Panel A generated using BioRender.]]<br> | ||
| + | |||
| + | When the SMM slides are imaged, the scanner exposes each slide to excitation light specific to the fluorophores used in the experiment. As shown in the figure above, two fluorophores were used to evaluate small molecule binding to the protein of interest. The green spots represent locations on the SMM slide where fluorescein was printed. Fluorescein is a fluorescent dye that emits at 532 nm and is used for alignment purposes. Correct alignment is critical to knowing which small molecules are in which spots on the slide. Red spots are indicative of small molecules that are bound by the protein of interest. The signal is due to an Alexa Fluor 647 anti-His antibody that emits at 635 nm. | ||
| + | |||
| + | During imaging, the scanner detects the intensity of the emitted fluorescence light to generate an image that can be analyzed. The intensity and position of the 'red' signal associated with putative small molecule binders is what will be assessed in the next laboratory session. This analysis will provide a list of 'hits' that can be used in future studies to identify drug candidates. | ||
| + | |||
| + | '''Analyzing SMM results''' <br> | ||
| + | |||
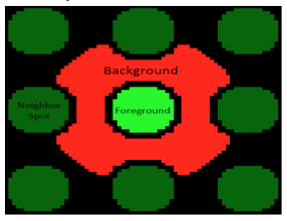
| + | [[Image:Sp20 M1D6 background, foreground.png|right|800px|thumb]]The microarrayer reads the fluorescence signals emitted from the surface of the SMM slide at two excitation wavelengths. As noted previously, the 532 nm wavelength was used to excite fluorescein, which was printed in an 'X' pattern to assist with alignment. The 635 nm wavelength was used to excite Alexa Fluor 647-conjugated anti-His antibody; which would be associated with the protein of interest bound to a small molecule on the slide. A hit denotes a spot on the slide that emits a red fluorescence signal significantly higher than the background fluorescence level. In terms of protein binding, a hit denotes that the protein of interest is bound to a small molecule and is therefore localized to a specific position on the slide. You will analyze the fluorescence signal collected by the microarray scanner using a value termed the robust z-score. | ||
| + | |||
| + | The robust z-score differentiates signal from noise by providing a value that represents the intensity of a signal above background. In the case of the SMM experiment, the intensity of a fluorescent signal above the background fluorescence is calculated. To do this the fluorescence emitted across the entire slide is grouped to define the Median Absolute Deviation (MAD), which is is a measure of the variability of a univariate dataset. Though beyond the scope of this class, the equation for calculating the robust z-score assigns a value for how much more intense the fluroescent signal at a spot is over background. The higher the value, the more different the signal from background. After putative binders are identified via the robust z-score, the data are examined by-eye. | ||
| + | |||
| + | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | ||
| + | |||
| + | *What is a downside of using this approach to identify small molecules that bind a specific target? | ||
| + | *What is an upside of using this approach to identify small molecules that bind a specific target? | ||
| + | |||
| + | ===Part 3: Draft discussion section for Research article=== | ||
As the final section of your Research article, you will write a formal Discussion that summarizes the key findings and states the implications of your research. Use the homework you completed for today to draft the Discussion for your Research article. | As the final section of your Research article, you will write a formal Discussion that summarizes the key findings and states the implications of your research. Use the homework you completed for today to draft the Discussion for your Research article. | ||
Latest revision as of 15:58, 13 November 2023
Contents
Introduction
Research takes time! The work that is reported in a published article is often a culmination of years of study by multiple investigators. Because of this it is very difficult to complete an entire project in the time span of a single semester. The work you completed this semester is part of an ongoing research project that started even before your previous classmates.
Previous work In work completed by the Fall 2022 class, students screened a library of small molecules that are chemically altered versions of FK506 which is known to bind to PfFKBP35 and the human ortholog, FKBP12. The goal of the initial screen was to examine the binding of each small molecule to PfFKBP35 and to FKBP12 in an effort to identify molecules that could potentially be used to target PfFKBP35 preferentially over FKBP12. From this experiment, the four small molecules used in your research were identified.
Your work The research goal for this module was to further study small molecules that were previously identified to bind to PfFKBP35. To better characterize the binding parameters of each of the four identified small molecules to PfFKBP35, you examined binding across different concentrations of small molecule. The Tm for PfFKBP35 across a range of small molecule concentrations can be used to confirm that binding is occurring and can also give some insight into the strength of the binding. If the small molecule binds to FfFKBP35, then a shift in the Tm will be observed and can be quantified by subtracting the Tm of PfFKBP35 without small molecule. The strength of binding can qualitatively be addressed by considering the concentrations of small molecule needed to cause a shift in the Tm. If a shift in Tm for a small molecule is observed at only high concentrations, then perhaps the strength of binding is not as good as for another small molecule that causes a shift in Tm at lower concentrations.
Future work There are several next steps that can be taken from your work. An obvious experiment is to test the small molecules used in your work with FKBP12 to confirm that the binding to PfFKBP35 is preferential across a range of concentrations of small molecule. If a small molecule passes this step, then perhaps modifications can be made to further improve the affinity and specificity to PfFKBP35. If a small molecule doesn't emerge as promising, then perhaps it is time to restart the screening process to identify new molecules.
Protocols
Part 1: Consider modifications that could improve binding to PfFKBP35
One method used to identify small molecules that bind to a specific target is to rationally design small molecules based on the structure information. If the structure of the protein target is known, then this information can be used to build a molecule that binds. In reality this is a very challenging task! To increase the chances of success using rational design, a scaffold molecule that is a known binder can be used. This is the approach that was used to develop the initial library of molecules that was screened by students in Fall 2022. The library was built by chemically modifying FK506, which is known to bind to PfFKBP35. This approach is only useful when a known small molecule binder is known. In addition, using this approach is not be useful in discovering molecules that bind outside of a known binding pocket or active site.
One method for assessing what functional groups are potentially important in protein-small molecule binding is to visually inspect known small molecule binders for common features / structures. As shown in the image below, common shapes can be identified between the small molecules.
In your laboratory notebook, complete the following:
- For the small molecules that showed binding from the class DSF results,
- Are there any common features present?
- If there are common features, do these features resemble the functional groups you hypothesized would promote binding to PfFKBP35 in the exercise completed on M2D4?
- What might this suggest about the potential binding site(s) on PfFKBP35?
- How might you make modifications to the small molecules / features to improve binding to PfFKBP35?
- What is a downside of using this approach to identify small molecules that bind a specific target?
- What is an upside of using this approach to identify small molecules that bind a specific target?
Part 2: Consider other methods for small molecule discovery
In contrast to rational design, another method used to find small molecules that bind a protein of interest is to use a high-throughput screen, such as the small molecular microarray (SMM). Though we did not use this method as part of this module, the technology and the data analysis workflow will be briefly described below as this could be an alternative strategy to the approach used in this module. This technology enables researchers to screen thousands, to hundreds of thousands, of small molecules in an unbiased manner using commercially available libraries.
Preparing the small molecule microarray
An SMM screen requires the covalent attachment of a library of small molecules to a glass slide. The library is meant to broadly sample the drug-like chemical space (i.e. all possible chemical structures that have drug-like physical properties) and contains about 50,000 small molecules. Some libraries are much smaller, while many pharmaceutical companies possess high-throughput screening (HTS) collections of millions of compounds. Because this chemical space is very large, it’s difficult to generalize any single chemical reaction for this attachment that can be applied to all small molecules. This method uses a “one-size-fits-most” approach, where the glass slide is functionalized with a broadly reactive electrophile capable of reacting with nucleophiles present in most drug-like small molecules, such as alcohols or amines. Many small molecules contain multiple nucleophiles suitable for attachment. In this case, manufacturing will result in a mixture of attachment sites. It’s important to remember that attachment to the glass slide constrains the possible orientation of the protein-small molecule interaction; some orientations are not possible because the glass slide and linker are in the way.
First, a glass slide with exposed amines across the surface and attach a short PEG (polyethylene glycol) linker. To the end of this PEG linker, an isocyanate group is attached. Isocyanate, or R-N=C=O, is a resonant structure, and a partial positive charge is stabilized on the carbon atom. This carbon atom is electrophilic, and small molecules with nucleophiles will react here. It is estimated that about 70% of drug-like small molecules are amenable to this reaction, and the library is filtered to contain only these molecules.
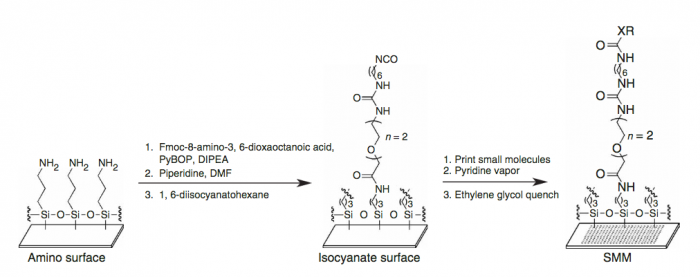
The compound library is dissolved in DMSO and stored in 384-well plates. To dispense the compounds onto our functionalized glass slide, a robotic arm with a set of 48 metal pins is used to transfer the compounds to the glass slide. Each metal pin has a small slit in the end, and capillary action is used to precisely withdraw and dispense consistent volumes. When the pins touch the glass slide, the compound in DMSO is dispensed into a small circle of approximately 160 micron in diameter. Each pin prints one compound in two different locations on each slide, and then the pins are washed in water and DMSO. This process is repeated for each compound, resulting in our final microarray. The microarray is divided into 48 subarrays, and each subarray corresponds to one pin and contains 256 discrete spots. Within each subarray, we print a set of fluorescent compounds in the shape of an ‘X’ so that we can precisely determine where each spot is printed. After the compounds react, we quench the surface so that no electrophiles remain. This results in our final microarray; a collection of approximately 12,000 discrete spots displaying one compound each.
Performing an SMM screen
To see the steps used to screen small molecules, please watch the video tutorial linked here: [SMM Screen]. The steps are detailed below so you can follow along!
Scanning SMM slides
After the printed SMM slides are incubated with purified protein, the next step is to check which of the small molecules, if any, you screened may be able to bind the protein of interest. To do this the slides are imaged using a Genepix microarray scanner. The scanner measures the fluorescence signal emitted from the slide at two wavelengths: 532 nm and 635 nm. The goal for today is to familiarize you with how the SMM slides are scanned and imaged. These images are the raw data that will be used to identify putative small molecule binders.

When the SMM slides are imaged, the scanner exposes each slide to excitation light specific to the fluorophores used in the experiment. As shown in the figure above, two fluorophores were used to evaluate small molecule binding to the protein of interest. The green spots represent locations on the SMM slide where fluorescein was printed. Fluorescein is a fluorescent dye that emits at 532 nm and is used for alignment purposes. Correct alignment is critical to knowing which small molecules are in which spots on the slide. Red spots are indicative of small molecules that are bound by the protein of interest. The signal is due to an Alexa Fluor 647 anti-His antibody that emits at 635 nm.
During imaging, the scanner detects the intensity of the emitted fluorescence light to generate an image that can be analyzed. The intensity and position of the 'red' signal associated with putative small molecule binders is what will be assessed in the next laboratory session. This analysis will provide a list of 'hits' that can be used in future studies to identify drug candidates.
Analyzing SMM results
The robust z-score differentiates signal from noise by providing a value that represents the intensity of a signal above background. In the case of the SMM experiment, the intensity of a fluorescent signal above the background fluorescence is calculated. To do this the fluorescence emitted across the entire slide is grouped to define the Median Absolute Deviation (MAD), which is is a measure of the variability of a univariate dataset. Though beyond the scope of this class, the equation for calculating the robust z-score assigns a value for how much more intense the fluroescent signal at a spot is over background. The higher the value, the more different the signal from background. After putative binders are identified via the robust z-score, the data are examined by-eye.
In your laboratory notebook, complete the following:
- What is a downside of using this approach to identify small molecules that bind a specific target?
- What is an upside of using this approach to identify small molecules that bind a specific target?
Part 3: Draft discussion section for Research article
As the final section of your Research article, you will write a formal Discussion that summarizes the key findings and states the implications of your research. Use the homework you completed for today to draft the Discussion for your Research article.
Remember that the Results and Discussion information will be separate in this more formal writing assignment. Use the questions below to help you decide which details should be included where in your text.
Next day: Brainstorm ideas for Research Proposal presentation