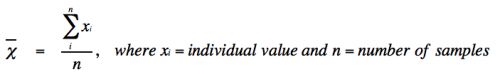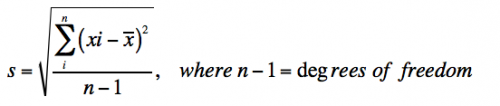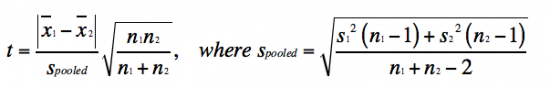20.109(F18):Practice statistical analysis methods and complete data analysis (Day7)
Contents
Introduction
Today is the final laboratory session for Module 1! You have completed all of the bench work for your research; however, there is still data analysis to complete for your experiments. In addition to plotting and normalizing the data, you will complete statistical analysis to determine the significance of your results.
Statistics are mathematical tools used to analyze, interpret, and organize data. The specific tools that you will use are confidence intervals (CI) and the Student's t-test. To begin, review the following definitions:
- Mean (or average) is defined as:
- With infinite data, the mean (χι) approaches the true mean (μ).
- Standard deviation measures the variation in the data and is defined as:
- With infinite data, the standard deviation (s) approaches the true standard deviation (σ).
Because standard deviation is only justified when sufficient data have been collected to generate a normal curve, you will use confidence intervals to report the likelihood that your results predict the true mean. A confidence interval is a defined interval that is calculated to define the true mean to a specified level of confidence. Simply, it is possible to define a range in your data set that likely contains the true mean based on the calculated mean.
- Confidence interval is defined as:
In your data, you should use the CI to generate error bars due the low n. Be sure to report which confidence level was used to calculate the intervals reported. So, what does this all mean in regard to the data you will report? As an example, if the calculated χι of a data set equals 80 au there is a 95% chance the μ is between 50 au and 110 au, where au = arbitrary units. And how does this relate to s? If you know the μ, the σ represents a 68% confidence interval.
Lastly, you will use Student's t test to report if your data are statistically different between treatments.
- Student's t test is defined as:
The value you calculate with the Student's t test equation is referred to as tcalculated. This tcalculated value is compared to the ttabulated value in the the t table, according to the appropriate n - 1 using the p-value for the two-tailed distribution (which assumes that you do not know how the data will shift). If the tcalculated value is greater than the ttabulated, then the data sets are significantly different at the specific p-value. So, what does this all mean in regard to the data you will report? As an example, if the tcalculated for a data set with n - 1 = 10 is 3 (given that the ttabulated is 2.228), then the data sets are different with a p-value ≤ 0.05. Which means that there is less that a 5% chance that the data sets are the same.
Part 1: Visualize H2AX assay results
- Make sure to have TBS solution available before you start. Aspirate the secondary antibody solution off the coverslip and immediately add 150 μL of TBS. Do not let the coverslips dry out during this process.
- To complete the post secondary wash, add 150 μL of TBS per coverslip, let incubate at room temperature for 3 min covered, then aspirate.
- Repeat this step twice.
- Obtain glass slides from the front laboratory bench and label your slides with all of your experimental information and group name, add 5 μL of mounting media to the slide.
- Aspirate the final TBS wash and using tweezers place the coverslip cell-side down on the mounting media "spot" on the microscope slide. Try your best to avoid bubbles by slowly placing the coverslip over the mounting media.
- The cell-side of the coverslide is the side that was facing up in the staining chamber.
- Complete Steps #3-4 for coverslips from all of the coverslips you stained.
- Alert the teaching faculty when all of your microscope slides are ready and you will be escorted to the microscope in the Engelward laboratory.
Part 2: Practice statistical analysis
Review these data from an experiment where cells were exposed to increasing amounts of radiation. Your goal is to determine if a statistically significant amount of DNA damage was induced. For the purpose of this exercise, the values in the spreadsheet are in arbitrary units of 'DNA damage', where the higher numbers indicate more damage.
When interpreting the statistics, consider how you may use the information to convince someone that the DNA damage was significant. You may find this spreadsheet, originally created by Prof. Bevin Engelward and modified by the 20.109 staff, helpful for this exercise. At a minimum, you should post a bar plot of the data with 95% confidence intervals and indicate if there is a statistically significant difference (i.e. provide a p-value) between conditions in your Benchling notebook.
Part 3: Complete data analysis
Use the tools above to analyze the data for your genomic instability (CometChip) and sub-nuclear foci (gamma-H2AX) experiments. The figures / analyses in your Data summary should include measures of variability (i.e. confidence intervals) and significance (i.e. p-values).
Complete H2AX image analysis
Please obtain your images from the instructors. Three sets of images (i.e. image stacks) were taken per condition, and each image stack contains images from two channels--DAPI (blue) and FITC (green). Remember that the secondary antibody you used for the gamma-H2AX staining was conjugated to an Alexa488 fluorophore, which emits green light. For each image stack, you will use ImageJ to 1) identify the location of the nuclei using the DAPI channel and 2) quantify the total gamma-H2AX fluorescence in the FITC channel at those specified nucleus locations.
Next day: Complete in silico cloning