Difference between revisions of "20.109(F15):Battery assembly and testing (Day5)"
Noreen Lyell (Talk | contribs) (→Introduction) |
Noreen Lyell (Talk | contribs) (→Introduction) |
||
| Line 4: | Line 4: | ||
Today you will assess the data to determine how well your battery performed. Previously, you prepared a battery electrode, specifically a cathode, from Fe(III)-phage nanowires that you combined with carbon. The redox properties of this active material determine the operating voltage, while other properties influence the capacity (how long the battery 'lasts' under a given current load) and rate capability (how quickly the battery is discharged or charged). Capacity and rate capability can be improved by making materials very small or by incorporating conductive metals into the matrix into the material. The goal of your research is to assess how the amount of phage used in biomineralization affects capacity. | Today you will assess the data to determine how well your battery performed. Previously, you prepared a battery electrode, specifically a cathode, from Fe(III)-phage nanowires that you combined with carbon. The redox properties of this active material determine the operating voltage, while other properties influence the capacity (how long the battery 'lasts' under a given current load) and rate capability (how quickly the battery is discharged or charged). Capacity and rate capability can be improved by making materials very small or by incorporating conductive metals into the matrix into the material. The goal of your research is to assess how the amount of phage used in biomineralization affects capacity. | ||
| − | + | The '''theoretical capacity''' of a battery is the quantity of electricity involved for the electro-chemical reaction within the battery. For our Fe(III)-phage batteries, the theoretical capacity is 178 mA*h/g, a value based on the cathode material - FePO<sub>4</sub>. Using this information, we can calculate the "'''loading factor'''" for the Galvanostat to be 54.9 mA/g (theoretical capacity divided by the time used to charge/discharge). This is the amount of current to use for a 10 hour discharge. The discharge time is selected by the researcher. Slower discharge times are better as diffusion of the solvents is increased making it more likely that your battery will reach it's theoretical capacity. | |
| − | The '''theoretical capacity''' of a battery is the quantity of electricity involved for the electro-chemical reaction within the battery. For our | + | |
| − | + | ||
| − | [[Image: | + | To calculate the current needed to fully charge or discharge your battery, you need to account for the mass of your cathode. The mass of every electrode will be a little different, so the charge/discharge rates will be slightly different. But as an example, if your battery cathode is precisely 1 mg of active material, then to discharge it in 10 hours we'd have to apply a negative current of -54.9 μA. |
| + | <br><center>1 mg active material x 178 mA*h/g x 1 g/1000 mg x 1/10 h = 54.9 μA</center><br> | ||
| + | It is important to remember note that your cathode consists of more than just the active material. Remember that when you prepared your cathode material you used 70% Fe(III)-phage nanowires, 25% Super P, and 5% PTFE...in addition your Fe(III)-phage nanowires are ~10% phage. To account for this, you need to adjust the weight of your cathode weight by multiplying by 0.63. Be sure to know how this value was determined! | ||
| + | |||
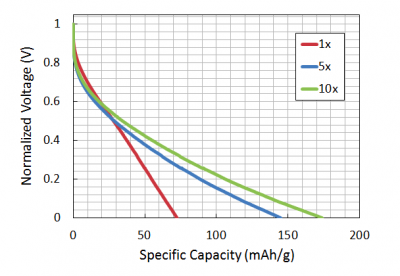
| + | [[Image:Fa15 20.109 example capacity data plot.png|thumb|right|400 px|Plot illustrating how the of amount of phosphate influences capacity when phage quantity is held constant.]]For a real world example, take a look at your cell phone battery. If you have a typical LiCoO<sub>2</sub> battery you will notice that it has a '''voltage''' (3.7 V) and a '''capacity''' (e.g. 710mA*h for the Motorola MOTORAZR V3r battery). Given that LiCoO<sub>2</sub> has a theoretical capacity of 130 mA*h/g, it's possible to back calculate the amount of active material as 5.46 g (try it!). Motorola determined the capacity and voltage for their cell phone batteries by testing them on a Galvanostat. This machine applies either positive or negative current to charge or discharge the batteries, identical to the procedure used to test your battery. By attaching a battery to a Galvanostat and applying a constant current, the battery will charge in a given time interval. By applying a negative current it's possible to discharge the battery in that same amount of time. A discharge graph, such as the one shown here, plots voltage ''vs.'' capacity discharged. The flatter the curve the better, since this means voltage remains constant as the battery is used. | ||
==Protocols== | ==Protocols== | ||
Revision as of 20:08, 1 December 2015
Introduction
Today you will assess the data to determine how well your battery performed. Previously, you prepared a battery electrode, specifically a cathode, from Fe(III)-phage nanowires that you combined with carbon. The redox properties of this active material determine the operating voltage, while other properties influence the capacity (how long the battery 'lasts' under a given current load) and rate capability (how quickly the battery is discharged or charged). Capacity and rate capability can be improved by making materials very small or by incorporating conductive metals into the matrix into the material. The goal of your research is to assess how the amount of phage used in biomineralization affects capacity.
The theoretical capacity of a battery is the quantity of electricity involved for the electro-chemical reaction within the battery. For our Fe(III)-phage batteries, the theoretical capacity is 178 mA*h/g, a value based on the cathode material - FePO4. Using this information, we can calculate the "loading factor" for the Galvanostat to be 54.9 mA/g (theoretical capacity divided by the time used to charge/discharge). This is the amount of current to use for a 10 hour discharge. The discharge time is selected by the researcher. Slower discharge times are better as diffusion of the solvents is increased making it more likely that your battery will reach it's theoretical capacity.
To calculate the current needed to fully charge or discharge your battery, you need to account for the mass of your cathode. The mass of every electrode will be a little different, so the charge/discharge rates will be slightly different. But as an example, if your battery cathode is precisely 1 mg of active material, then to discharge it in 10 hours we'd have to apply a negative current of -54.9 μA.
It is important to remember note that your cathode consists of more than just the active material. Remember that when you prepared your cathode material you used 70% Fe(III)-phage nanowires, 25% Super P, and 5% PTFE...in addition your Fe(III)-phage nanowires are ~10% phage. To account for this, you need to adjust the weight of your cathode weight by multiplying by 0.63. Be sure to know how this value was determined!
Protocols
Reagents list
Next day: Research proposal presentations
Previous day: TEM